| المجلد 3 , العدد 10 , ذي الحجة 1426 - كانون الثاني (يناير) 2006 |
| |
| AQ4N: a Promising Bioreductive Drug for Cancer |
| AQ4N: دواء حيوي ارجاعي واعد للسرطان |
| Kenan Alkhoury |
| د. كنان الخوري |
| The Institute of Cancer Therapeutics, University of Bradford, England |
| الملخص Abstract |
AQ4N هو دواء جديد من مجموعة الأدوية الحيوية الارجاعية وهو غير قادر على الدخول ضمن الـ DNA لكنه يُستقلب تحت شروط عوز الأكسجين بواسطة السيتوكروم P450 إلى شكل ثابت AQ4. يُعد الـ AQ4 مثبطاً للـ DNA توبوايزوميراز II ويمكن أن يسبب انكساراً في طيقان الـ DNA.
هدفت هذه الدراسة إلى تقييم AQ4N وشكله المُرجع AQ4 وسمّيتهما في عدة خطوط خلوية مختارة عشوائياً، بالإضافة إلى دراسة توزعهما ضمن الخلية.
تمت الدراسة AQ4N/AQ4 ضد ثلاثة خطوط خلايا مختلفة: السرطانة الغدية المبيضية البشرية (A2780) وسرطان القولون البشري (BE و DLD-1).
أظهرت النتائج أن طليعة الدواء AQ4N لم تنفذ إلى النواة ولم تظهر تأثيرات سميّة تجاه جميع خطوط الخلايا المستخدمة جميعها. في حين نفذ العقار AQ4 إلى النواة وكانت سميته الخلوية تجاه الـ A2780 أكبر بحوالي 24 ضعفاً منها تجاه الـ BE.
الـ AQ4N هو من الأدوية الحيوية الارجاعية الواعدة مع سمية خلوية كبيرة وهو شكله المُرجع AQ4 واستجابت الأورام المختلة لهذا العقار بشكل مختلف.
|
AQ4N is a novel bioreductive drug which is unable to intercalate into DNA. It is metabolized under hypoxic conditions, by cytochrome P450, to a stable form AQ4. AQ4 is considered as DNA topoisomerase II inhibitor and can cause DNA strands break.
This study aimed to evaluate AQ4N and its reduced form AQ4, their cytotoxicity against randomly chosen cell lines, besides studying their distribution within the cell.
AQ4N/AQ4 have been tested against three different cell lines, human ovarian adenocarcinoma (A2780) and human colon cancer (BE and DLD-1).
The results showed that the prodrug AQ4N did not penetrate into the nucleus and did not show cytotoxic effects in all used cell lines. However, the drug AQ4 penetrated into nucleus and its cytotoxicity against A2780 was about 24 fold more than its cytotoxicity in BE.
AQ4N is a potential bioreductive drug with a considerable cytotoxicity of its reduced metabolite AQ4 and different tumours respond differently to AQ4.
|
Introduction
Bioreductive Drugs
|
Bioreductive drugs are defined as small molecule prodrugs which are inactive in their parent form but become toxic when enzymatically reduced under hypoxic conditions (Rauth et al.,1998). Such agents have the potential to turn tumour hypoxia to our advantage by exploiting it to activate prodrugs within tumours. The selectivity of such drugs is determined by two main properties: (i) the ability of oxygen to reverse the activation process and (ii) elevated levels of reductases in tumours. The key requirement of prodrug cancer therapy is the selective activation of the drug in the tumour environment (Loadman et al., 2001). The principle behind this method is to administer non-toxic forms of the drug systemically which would be activated to the toxic form only in tumour tissues. The activation step makes use of some unique physiological, metabolic, or genetic differences between normal and tumour tissues. One of the most important differences is the presence of hypoxic cells in solid tumours (
Denny, 2004). Since the late 1980s, two new approaches were developed: ADEPT, in which a prodrug activating enzyme is targeted to a tumour using a tumour-antigen-associated antibody (Bagshawe, 1993); and GDEPT, in which a gene encoding the activating enzyme is targeted for expression in malignant cells under control of a tumour-specific promoter (Connors, 1995; Roth and Cristiano, 1997).
The properties of optimal bioreductive drug include stability and an ability to penetrate the extravascular space of the site of activation. The selection of the activating enzyme and prodrug is very important for the successful treatment and can be achieved by the selection of the best possible prodrug-activating enzyme, “enzyme profiling” for individual tumour, or by determining the heterogeneity in hypoxic cell fractions between individual tumours. Many approaches were developed for the delivery of the activating agents by using gene therapy e.g. the use of viruses and liposomes. There are also newly developed techniques such as the use of hypoxia-dependent bacteria spores as vectors (Lemmon et al., 1997).
Many studies have been done on such compounds with a limited success and the clinical trials were confined to four types of compounds (Denny, 2004): Quinones e.g. EO9, Nitro aromatic e.g. CI 1010, Aromatic N-Oxides e.g. TPZ and Aliphatic N-Oxides e.g. AQ4N which is our area of study.
|
| AQ4N |
|
1,4-bis{[2-(dimethylamino) ethyl] amino}-5,8 dihydroxyanthracene-9,10-dione bis-Noxide (AQ4N) is a novel bioreductive drug developed by Patterson (Patterson, 1993). The prodrug AQ4N, which is unable to intercalate into DNA, is metabolized in hypoxic cells by cytochrome P450s (CYPs) (McErlane et al., 2005) to a stable form AQ4 which is able to intercalate into DNA; this prodrug is considered to be the first P450-activated bioreductive drug in clinical trials (McFadyen et al., 2004). AQ4N is usually prepared by oxidation of the bisamine precursor (AQ4) (Lee and Denny, 1999). The reduction of the two N-Oxides generates the tight DNA binder and topo II poison AQ4, which is analogue of mitoxantrone (Smith et al., 1997). The main principle is that the two amino N-oxides are deactivated DNA binding agents, and the twoelectron N-oxide reduction occurring in hypoxic tumour cells converts them to an active form (Smith et al., 1997). The major target of this drug is DNA topoisomerase II which can cause
transient DNA double strand breaks and hence DNA structure changes. This drug is designed to preferentially kill the hypoxic cells and the neighbouring cells. When the stable active drug is formed from the prodrug, it will stay active toward aerobic cells and may diffuse from hypoxic regions (chronic hypoxia) to act on surrounding aerobic cells in the tumour (Rauth et al., 1998).
|
| Principle of the reaction of AQ4N: |
The reaction by which AQ4N gives rise to AQ4 (figure 1) is mediated by a number of cytochrome P450s (CYPs) including CYP3A4 and CYP2B6. (McFadyen et al., 2004) demonstrated that the presence of CYP3A4 increased the metabolism of AQ4N and significantly increased the killing power of this cytotoxin. This conversion has a great effect:
AQ4N: N-oxides are electrically neutral at physiological pH; AQ4N has a negligible affinity for DNA and is non-cytotoxic for the cells. Clinical studies have shown AQ4N to be well tolerated without serious toxicity (Benghiat, 2005).
AQ4: In contrast, the alkylaminoalkyl groups of AQ4 are protonated at physiological pH and bind strongly to DNA by interaction with phosphate residues. AQ4 is stable chemically and acts as DNA affinic topoisomerase II inhibitor (Wilson et al., 1996). Inhibition of topoisomerase II inhibitor by AQ4N results in synchronizing cell growth. Thus AQ4N will sensitize tumours to the repeated courses of radiotherapy (Loadman et al., 2001).
The primary reduction of AQ4N will generate the intermediate compound AQ4M (figure 1).
Further reduction of AQ4M will generate the stable form AQ4 (Chinje et al., 2004). Williams and colleagues suggested that the role of cytochrome p450 protein in AQ4N metabolism may be more important in the conversion of AQ4M to AQ4 rather than the initial conversion of AQ4N to AQ4M (Williams et al., 2004). Thus it could be said that for AQ4N to be effective, there is a need to have both hypoxia and a sufficient level of the appropriate CYP isoforms present in the tumour (Patterson et al., 1999; Raleigh et al., 1998). A study by (McErlane et al., 2005) showed that the enhancement of CYP levels by using gene-directed enzyme prodrug therapy (GDEPT) increased the anti-tumour effect of the combination of AQ4N with radiation due to increasing of the bioreduction of AQ4N (McKeown et al., 1995).
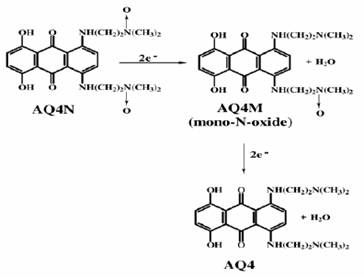
Figure 1: the reduction of AQ4N to AQ4 (Swaine et al., 2000)
The reduction to AQ4 results in increasing this affinity several folds due to cationic charge of the metabolite allowing interaction with the sugar phosphate backbone of the DNA (Smith et al., 1997). Relatively high doses of AQ4N are well tolerated because the toxic form is only released in hypoxic tissue.
The metabolic conversion of AQ4N to AQ4 is dependent on decreased oxygen tension. AQ4N is unique in that when converted into the active form, this form is unaffected by tumour reoxygenation (Hejmadi et al., 1996). That means AQ4N will continue to act even after an acute hypoxic episode. Due to the high binding affinity of the metabolite AQ4 it is unlikely to diffuse out of the hypoxic regions of tumours where it was reduced. Thus low plasma levels of AQ4 are to be expected (Loadman et al., 2001).
|
Aims of the Project
This project was aimed to identify the pharmacological properties of AQ4N and its reduced form AQ4 through:
1. Chemosensetivity study of these compounds against randomly chosen cell lines.
2. Detection the IC50 of these compounds.
3. The distribution of these compounds within the cell using confocal microscopy.
Materials and Methods
All chemicals and medium were obtained from Sigma (SIGMA-ALDRICH® company, Ltd Irvine, Ayrshire KA12 8NB, UK). AQ4N and its derivatives were obtained from Prof Patterson and Dr Pors, University of Bradford.
Plastics: the flasks were obtained from (Corning®, Surrey GU3 1LR, UK), universal tubes from (Sarstedt®, Leicester LE4 1AW UK), and the centrifuge tubes from (Greiner Bio-One Ltd, Gloucestershire GL10 3SX, UK).
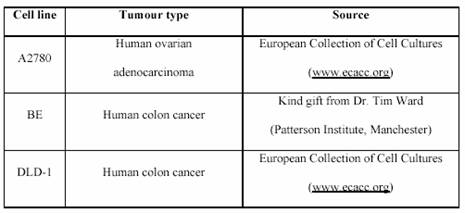
The cell lines used are listed in Table 1.
Cell line Tumour type Source
A2780 Human ovarian adenocarcinoma European Collection of Cell Cultures
(www.ecacc.org)
BE Human colon cancer
Kind gift from Dr. Tim Ward
(Patterson Institute, Manchester)
DLD-1 Human colon cancer
European Collection of Cell Cultures (www.ecacc.org) All cell lines were routinely grown as a monolayer cultures in 75 cm2 cell culture flasks in an atmosphere of 5% CO2 and 95% air at 37?C. They were maintained in “complete” Roswell Park Memorial Institute (RPMI) 1640 culture medium.
Several methods have been used through this study; cells were passaged to a new flask every few
days with a maximum number of passaging of 11 times; the chemosensetivity assay MTT were used to detect the cytotoxicity of the used compounds against the cell lines; the Confocal microscope was used to study the distribution of the compounds within the cells and detect their location.
Table 1: The origin of the used cell line panel.
 |
| Results |
|
Chemosensetivity of AQ4N/AQ4
The response of two different cell lines, A2780 and BE, were tested against the drug AQ4 and its prodrug AQ4N through the MTT assay and each test was conducted in triplicate (figure 2).
The IC50 of the drug AQ4 was calculated for each cell line. IC50 for A2780 was 0.024 µM and for BE was 0.6 µM. However, the IC50 of the prodrug AQ4N was over 10µM in both cell lines.
Drug distribution studies using confocal microscopy
The distribution of the three drugs and their derivatives were tested on the human ovarian adeno-carcinoma cell line (A2780) and human colon cancer cell line (BE). This involved incubation of cells in special chambers for overnight, with the drugs (10 mµ of each compound).
Confocal images were taken after 30 minutes and after one hour of incubation at 37 ?C. These photos are listed in the figures 3 to 5. Where confocal images of the controls were illustrated in figures 3.
It can be seen from figures (4-A and 5-A) that after 30 minutes of incubation AQ4 was still in perinucleur location in both A2780 and BE cell lines. However, after one hour AQ4 distribute into the nucleus in both cell lines (figures 4-B and 5-B). The prodrug AQ4N stayed in perinucleur location in both cell lines even after one hour of incubation at 37 ?C (figures 4-C, 5-C, 4-D, and 5-D).
Discussion
The chemo-sensitivity study
As shown in figures 2-A and C, the prodrug AQ4N didn’t have any cytotoxic effects against human ovarian adenocarcinoma cell line (A2780) and against the human colon cancer cell line; the IC50 was above 10µM in both cell lines. However, the metabolite AQ4 (figures 2-B and D) showed a considerable cytotoxicity in both cell lines with different values of IC50 (0.05µM for A2780 and 1.2µM for BE). It can be concluded that the prodrug AQ4N is inactive, while its metabolite AQ4 causes cell death after four
days of incubation. The difference between IC50 in the two cell lines show that AQ4 cytotoxicity in A2780 was 24 fold more than in BE cells. This is indicative of different responses from two different cell lines to AQ4. These different responses suggest differences in the enzymatic system between the two cell lines because the reduction of AQ4N to AQ4 depends on the availability of reducing enzymes e.g. cytochrome P450s (McFadyen et al., 2004). |
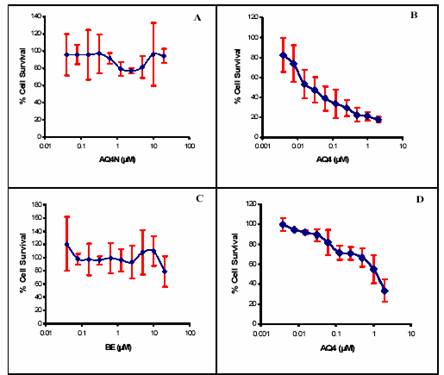
Figure 2: Drug response curve for AQ4N and AQ4 against the cell lines A2780/BE. (A): A2780 versus AQ4N (IC50 ،? 10µM), (B): A2780 versus AQ4 (IC50 = 0.05 ± 0.01 µM), (C): BE versus AQ4N (IC50 ،? 10µM), (D): BE versus AQ4 (IC50 = 1.2 ± 0.2 µM). Each data represents the mean ± S.D. (n=3). |
| After 30 minutes |
After one hour |
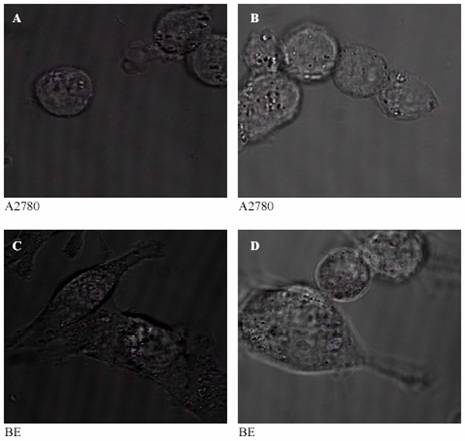
Figure 3: Confocal images of the controls: human ovarian adenocarcinoma cell line (A2780) after 30 minutes (A) and one hour (B) of incubation at 37 ?C and human colon cancer cell line (BE) after 30 minutes (C) and one hour (D) of incubation at 37°C. |
Therefore, the reaction of AQ4N to give AQ4 require number of cytochrome P450s (CYPs) including CYP3A4 and CYP2B6. It is unrealistic to expect all tumours to contain sufficient CYPs to give maximal reduction of AQ4N. So we can not expect AQ4N to have the same effect in all cell lines (McErlane et al., 2005). This result matches with previous study by (Jeffrey et al., 2004) who also suggested different response of different cell lines to AQ4.
|
| After 30 minutes |
After one hour |
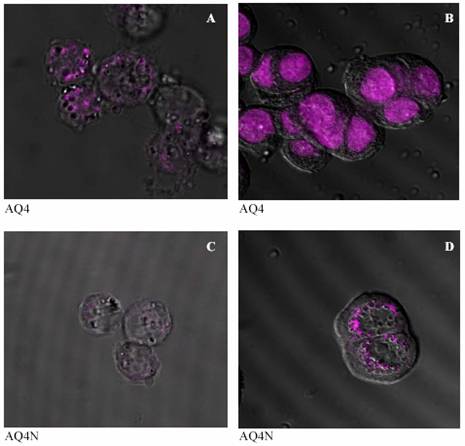
Figure 4: Confocal images of the distribution of AQ4 in human ovarian adenocarcinoma cell line (A2780) after 30 minutes and one hour (A and B respectively), and of AQ4N after 30 minutes and 1 hour (C and D respectively) of incubation at 37 °C.
| After 30 minutes |
After one hour |
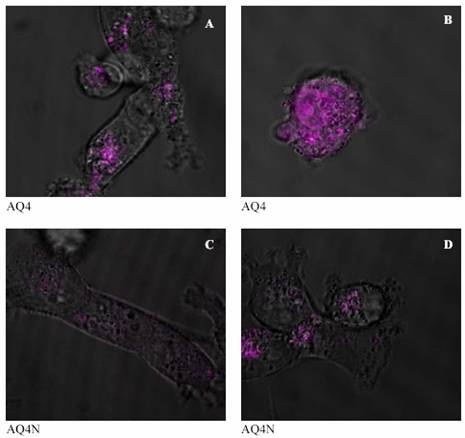
Figure 5: Confocal images of the distribution of AQ4 in human colon cancer cell line (BE) after 30 minutes and one hour (A and B respectively), and of AQ4N after 30 minutes and 1 hour (C and D respectively) of incubation at 37 °C. |
| |
The confocal study of the drugs distribution
It can be seen from figures 4 and 5 (C and D) that the prodrug AQ4N was located in the cytoplasm in both human ovarian adenocarcinoma and human colon cancer cell lines and did not penetrate into the nucleus, even after 60 minutes of incubation. The drug AQ4 after 30 minutes was located in perinucleur location (figures 4-A and 5-A). However, after 60 minutes, fluorescence could be seen in the nucleus suggesting a nucleus location of AQ4 in both used cell lines (figures 4-B and 5-B). The chemosensitivity experiment confirmed the negligible cytotoxicity of AQ4 and this could be attributed to its location. Being in the cytoplasm, it will not be able to intercalate with DNA and will not affect it. On the other hand, the nuclear location of AQ4 could be correlated with previous results from the cytotoxicity studies. This correlation suggests that AQ4 kills the cell by damaging DNA in the nucleus.
Conclusion
AQ4N is a potential bioreductive drug with a considerable cytotoxicity of its reduced metabolite AQ4. The drug may have different effects on different tumour cell lines. The negligible cytotoxicity of the bioreductive drug AQ4N and the considerable cytotoxicity of its active metabolite AQ4 the drug a potential candidate for the next stage of clinical trials which would aid in identifying the selectivity of this drug in different tumour cell lines.
Acknowledgment
This work was carried out in the Institute of Cancer Therapeutics in the University of Bradford, UK; I thank Dr. Phillips for the valuable help and supervision and Prof Patterson for supplying with AQ4N and AQ4.
|
References |
1-Bagshawe KD.
Antibody-directed enzyme prodrug therapy (ADEPT).
Adv Pharmacol; 24, 99-121. 1993.
2-Benghiat AS; Middleton WPM; Talbot D; Patterson LH; Ford SJ; Hayward C. and Loadman P.M
The use of pharmacokinetic and pharmacodynamic endpoints to
determine dose of AQ4N given with radiotherapy (RT).
Journal of Clinical Oncology, 23, 2005.
3-Chinje EC; Kong Z; Williams KJ; Telfer BA; Wind SN. and J. S.I.
Elevated inducible NOS activity in human fibrosarcoma HT1080 tumour cells enhances AQ4N reductive metabolism in vitro.
Paper presented at: American Association for Cancer Research (AACR) Annual Meeting (Orlando, Florida). 2004.
4-Connors TA.
The choice of prodrugs for gene directed enzyme prodrug therapy of cancer.
Gene Ther, 2, 702-709. 1995.
5-Denny WA.
The Design of Drugs that Target Tumour Hypoxia.
Australian Journal of Biochemistry, 57, 821-828, 2004.
6-Hejmadi MV; McKeown SR; Friery OP; McIntyre IA; Patterson LH. and Hirst DG.
DNA damage following combination of radiation with the bioreductive drug AQ4N: possible selective toxicity to oxic and hypoxic tumour cells.
Br J Cancer, 73, 499-505, 1996.
7-Jeffrey LC. Alvin Wong, Susan E. Alters, Peter A. Harris, Chris R. Dunk, John G. Curd, Robert L. Capizzi and Henner WD.
A novel N-oxide drug, AQ4N, has in vitro activity in lymphoma and leukemia cell lines and selectively targets lymphocytes and lymphatic tissues in vivo.
Paper presented at: American Society of Hematology (ASH).
Annual Meeting (San Diego, California). 2004.
8-Lee HH. and Denny WA.
A large-scale synthesis of the bioreductive drug 1,4-bis{[2-(dimethylamino) ethyl]amino}-5,8-dihydroxyanthracene-9,10-dione bis-Noxide (AQ4N).
The Royal Society of Chemistry 1, 2755–2758, 1999.
9-Lemmon MJ; van Zijl P; Fox ME; Mauchline ML; Giaccia AJ; Minton NP. and Brown JM.
Anaerobic bacteria as a gene delivery system that is controlled by the tumor microenvironment.
Gene Ther, 4, 791-796, 1997.
10-Loadman PM; Swaine DJ; Bibby MC; Welham KJ. and Patterson LH.
A preclinical pharmacokinetic study of the bioreductive drug AQ4N.
Drug Metab Dispos, 29, 422-426, 2001.
11-McKeown SR; Hejmadi MV; McIntyre IA; McAleer JJ. and Patterson LH.
AQ4N: an alkylaminoanthra-quinone N-oxide showing bioreductive potential and positive interaction with radiation in vivo.
Br J Cancer, 72, 76-81, 1995.
12-McErlane V; Yakkundi A; McCarthy HO; Hughes CM; Patterson LH; Hirst DG; Robson T. and McKeown SR.
A cytochrome P450 2B6 meditated gene therapy strategy to enhance the effects of radiation or cyclophosphamide when combined with the bioreductive drug AQ4N.
J Gene Med, 7, 851-859, 2005.
13-McFadyen MC; Melvin WT. and Murray GI.
Cytochrome P450 enzymes: novel options for cancer therapeutics.
Mol Cancer Ther, 3, 363-371, 2004.
14-Patterson LH.
Rationale for the use of aliphatic N-oxides of cytotoxic anthraquinones as prodrug DNA binding agents: a new class of bioreductive agent.
Cancer Metastasis Rev, 12, 119-134, 1993.
15-Rauth AM; Melo T. and Misra V.
Bioreductive therapies: an overview of drugs and their mechanisms of action.
Int. J radiation Oncology Biol Phys, 42, 755-762, 1998.
16-Raleigh SM; Wanogho E; Burke MD; McKeown SR. and Patterson LH.
Involvement of human cytochromes P450 (CYP) in the reductive metabolism of AQ4N, a hypoxia activated anthra-quinone di-N-oxide prodrug.
Int. J Radiat Oncol Biol Phys, 42, 763-767, 1998.
17-Roth JA. and Cristiano RJ.
Gene therapy for cancer: what have we done and where are we going?
J Natl Cancer Inst, 89, 21-39, 1997.
18-Smith PJ; Blunt NJ; Desnoyers R; Giles Y. and Patterson LH.
DNA topoisomerase II-dependent cytotoxicity of alkylaminoanthraquinones and their N-oxides.
Cancer Chemother Pharmacol 39, 455-461, 1997.
19-Swaine DJ; Loadman PM; Bibby MC; Graham MA. and Patterson LH.
High-performance liquid chromatographic analysis of AQ4N, an alkylamino-anthraquinone N-oxide.
J Chromatogr B Biomed Sci Appl, 742, 239-245, 2000.
20-Williams KJ; Edwin C; Chinje BA; Telfer NS; Wind IJ; Stratford PH. and Dunk C.
potentiation of chemoradio-therapy in human lung xenografts using the bioreductive
agent AQ4N.
Paper presented at: American Association for Cancer Research (AACR) Annual Meeting (Orlando, Florida), 2004.
21-Wilson WR; Denny WA; Pullen SM; Thompson KM; Li AE; Patterson LH. and Lee HH.
Tertiary amine N-oxides as bioreductive drugs: DACA N-oxide, nitracrine N-oxide and AQ4N.
Br J Cancer Suppl, 27, S43-47, 1996.
|
| |
| المجلد 3 , العدد 10 , ذي الحجة 1426 - كانون الثاني (يناير) 2006 |
|
|
|
