| المجلد 3 , العدد 4 , جمادى الأولى 1425 - تموز (يوليو) 2004 |
| |
| اللعاب البشري: سائل فيزيولوجي تشخيصي فعال |
| Human Saliva: An Effective Physiological Diagnostic Fluid |
| Drs. Abdo R. Jurjus1 and Elias Ilyia2 |
| 1Professor, Faculty of Medicine, American University of Beirut-MC, Beirut, Lebanon
2Director, Diagnos-Tech Laboratories, Seattle, USA |
| الملخص Abstract |
يعود البحث عن مشعرات للأمراض الجهازية في اللعاب إلى حوالي قرن من الزمن، لكن تزايد استخدام اللعاب كطريقة قيمة وغير باضعة في السنوات العشرين الأخيرة، وقد سجلت اختبارات السائل الفموي رسمياً في 3 حزيران 1996عندما قبلت إدارة الغذاء والدواء FDA إجراء اختباراً للإيدز على اللعاب.
يُعد اللعاب الأسهل من حيث طريقة الجمع من بين سوائل الجسم، وعلى الرغم من أن معظم اللعاب ماء (99%) ووظيفته الرئيسية هي التزليق، والبدء بعملية الهضم، فإنه يحتوي على مئات من المركبات الأخرى التي تشكل آليات دفاع محكمة عن ميناء الأسنان والتجويف الفموي، وتنتج الغدد اللعابية تقريباً 1 لتر يومياً من اللعاب مصلي ومخاطي القوام والذي يحتوي على إنزيمات، معادن، بروتينات مصلية، خلايا دموية، جراثيم، إفرازات الطريق التنفسي العلوي... . وإن غالبية الجزيئات التي توجد في الدم والبول توجد أيضاً في اللعاب بتراكيز 1/10 إلى 1/1000 من تركيزها بالدم.
هنالك طرق مختلفة لجمع اللعاب، وتُعد طريقة البصق إلى إناء هي الأكثر استخداماً. أما طرق المعايرة التي يمكن تطبيقها على اللعاب فكثيرة وأهمها المقايسة المناعية الشعاعية RIA، والطريقة المناعية الإنزيمية EMIT، وتقنية الطبقة الرقيقة TLC وغيرها.
وقد لوحظ إن اختبارات اللعاب في غاية الأهمية تشخيصياً كونها تعكس مستويات المواد الحيوية، بعض الأدوية، الهرمونات والوضع المناعي في السوائل النسيجية، فاللعاب يمكن اعتباره كمرآة للجسم. |
| Introduction |
The last 2 decades of the twentieth century witnessed remarkable advances in the health care technology. The applications of such advances left a dramatic impact on health at the preventive, diagnostic, management, and even rehabilitation levels. Among such innovations are the wide clinical applications of salivary testing. Although the search for salivary indicators of systemic disease is nearly a century old, the use of saliva as a valuable non-invasive diagnostic tool has increased only in the past 20 years. Actually, more than 3600 articles dealing with salivary diagnostic tests have been published since 1983. In the USA, saliva tests have FAD approval, Third Party Cost-Coverage, and COLA accreditation.
Saliva is a very important body fluid. This mineral-filled, protein-rich fluid helps people to eat, digest food, talk, combat tooth decay and oral infections, and maintain tooth structure. Saliva could even assist in the diagnosis-without a single needle prick-of many diseases, including: hepatitis, autoimmune diseases, metabolic and endocrine diseases such as thyroid, diabetes, malabsorption, reproductive or fertility pathologies, infectious diseases such as hepatitis, HIV and others, and certain cancers and cystic fibrosis.
The traditional biological samples for the qualitative and quantitative measurement of most drugs, hormones or others, are blood, plasma and urine. In recent years, saliva has attracted much attention, in particular among people interested in the determination of drug concentrations and hormone levels, who suggested that saliva might be substituted for plasma in the areas of pharmacokinetic studies, drug monitoring, and endocrinology. Actually, many substances and their metabolites are present in different concentrations in these media. The blood or plasma provides an estimate of the current circulating concentration of the analyte of interest. The urine permits measurement of the accumulated concentration of analytes since the last void of the bladder.
Saliva's popularity has suffered because it lacks “the drama of blood, the sincerity of sweat and the emotional appeal of tears”. (Mandel 1990). Sweat and tears, however, are difficult to obtain in sufficient quantities for routine testing, and urine will always lack the charisma of the other fluids. Saliva, by default, therefore, becomes the most favoured alternative to blood.
Officially, oral-fluid testing dates back to June 3, 1996, when the Food and Drug Administration approved the Epitope Corp's testing procedure for human immuno-deficiency virus (HIV).
Since then, the Epitope system has also been approved for cocaine and nicotine testing.
Use of new saliva procedure has been spreading rapidly because it provides insurance companies with convenient, safe and less expensive means to acquire laboratory data on their clients. |
| Salivary glands anatomy and physiology |
By definition a salivary gland is any cell or organ that releases a secretion called saliva into the oral cavity. In fact, the mucous membrane of the mouth and tongue contains many small salivary glands that open directly or indirectly via small ducts into the oral cavity. These glands include (Fig. 1):

Figure 1: Salivary glands
* Labial (in the lips).
* Buccal (in the cheeks).
* Palatal (in the palate).
* Lingual (in the tongue).
|
The parotid glands are serous glands (devoid of mucin) while the submandibular and sublingual glands contain both serous- and mucin-secreting cells.
Saliva is a mixture of salivary gland secretion, gingival fluid, cellular debris and microorganisms of the oral cavity. Mixed saliva consists mainly of the secretions of submandibular (65%), the Parotid (23%), and the sublingual (4%) glands. The remaining 8% is being provided by the minor numerous glands.
The volume of saliva secreted appears to be entirely a function of the activity of the secretory endpiece. The ductal system neither reabsorbs nor adds further water. The external carotid arteries heavily irrigate the submandibular and sublingual glands. |
| Composition of saliva |
More than 99% of saliva is water. However, the majority of molecules found in blood and urine are also present in saliva (in concentrations 1/10th - 1/1000th the blood concentration) (Table 1).
Constituents of saliva include electrolytes, proteins, enzymes, immunoglobulins, and a variety of exogenous and endogenous organic substances. The analytes may originate in the salivary glands or be transferred into the saliva from the plasma.
|
Table 1: Analytes levels in saliva and plasma.
 |
The pH and composition of saliva change with its flow rate and are influenced by both physiological and psychological factors. The normal saliva pH at 37 °C ranges between 6.5 and 7.2. Resting saliva pH could be as low as 5.9. In addition, stress or anxiety depress the flow of saliva and lower its pH, while increased flow rate elevates saliva pH to 7.4 – 7.6. |
| Analyte transfer into saliva |
Saliva is a natural ultrafiltrate of plasma. The mechanism of a substance’s transfer into saliva has important implications for its diagnostic usefulness. Substances may pass from plasma to saliva by moving through or between cells. (Intracellular v/s intercellular).
The intracellular movement may be by active transport (e.g. Lithium, penicillin, and methotrexate) or by passive diffusion, which depends upon the analyte’s, molecular weight, lipid solubility, degree of ionization, or by degree of protein binding (Fig. 2). |
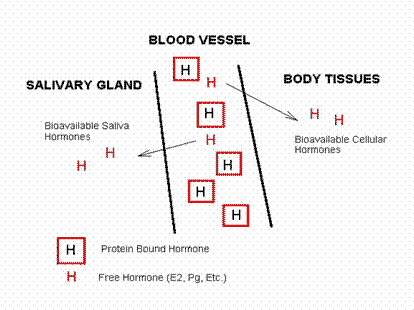
Figure 2: Analyte transfer into saliva |
Ultrafiltration is the most common mode of extracellular transport. Lipophilic, nonionized, and unbound analytes, including many drugs and unconjugated steroids, enter saliva by diffusion through the glandular epithelium.
Intracellularly transported analytes concentrations reflect the free analyte plasma concentration, while salivary concentrations of water-soluble substances transported extracellularly (e.g. Steroid conjugates through tight junctions between gland cells) do not necessarily parallel plasma concentrations. |
| The saliva-to plasma (S/P) ratio |
The S/P ratio indicates how readily a substance diffuses between saliva and plasma. The factors considered in calculating the ratio include substance pKa, degree of ionization, protein binding, and lipid solubility
Consideration of the predicted S/P of a substance is useful in assessing the potential for its detection and monitoring in saliva, however, calculated and observed S/P ratios may differ because of intersubject and intrasubject variability. Studies of the correlation between concentrations in blood and saliva have found examples of excellent concordance (e.g. ethanol, cortisol, lithium, theophylline, and antibodies to HIV) using highly sensitive methods of detection. However, a reliable ratio must exist between levels of a substance in plasma and levels of that substance in saliva. |
| Collection and analysis of saliva |
Mixed (whole) saliva is the only practical alternative for the collection of saliva samples on a patient basis. Spitting into a vessel is the most common collection method. However, many researchers have found it advantageous to further stimulate salivation.
a) Hints for the collection of saliva
1. Except for Cortisol, do not use plastic collection tubes (false low values), but glass tubes.
2. Except for Cortisol, do not use cotton or polyester rolls for saliva collection (false elevated values).
3. Do not use swabs with the addition of citric acid.
4. Do collect Saliva before brushing of teeth or at least 30 minutes after.
5. Do collect Saliva at least 30 minutes after eating or drinking.
6. Rinsing of the oral cavity with water just before saliva collection is recommended.
7. For saliva flow stimulation, chewing on an inert material like parafilm or a sugarless chewing gum is recommended.
8. Do not use even the slightest red saliva samples (because of blood contamination).
9. Saliva samples for steroid measurement may be stored for up to:
- 5 days at room temperature,
- 10 days at 2 - 8 °C,
- longer periods at -20 °C.
10. Freezing of the saliva samples and centrifugation just before application in the test is recommended.
b) Salivation
The secretion of saliva is controlled by the nervous system. Normally parasympathetic stimulation promotes continuous secretion of a moderate amount of saliva. On the other hand, sympathetic stimulation dominates during stress, resulting in dryness of the mouth. During dehydration, the salivary glands stop secreting saliva to conserve water. The touch and taste of food are potent stimulators of salivary glands secretions (nerves VII and IX). The smell, sight, sound or thought of food may also stimulate secretion of saliva. These stimuli constitute psychological activation and involve learned behavior. Salivation also occurs in response to swallowing irritating food or during nausea.
c) Pre-analytical manipulation
The usefulness of saliva determinations clearly depends also on the application of analytical procedures adequate for the assay in saliva. Once the samples have been collected, it is important that they be properly stored unless analyses are to be performed immediately.
Opinions differ as to the procedure to be followed for long-term storage of saliva samples, but most workers freeze the sample to -20° C, while some workers recommend centrifugation before freezing, and others recommend centrifugation after thawing and prior to analysis. A problem in saliva analysis is that smokers produce thick, very viscous saliva. By freezing all saliva samples at -40°C for at least 24 h before analysis, cellular components and suspended particles are effectively broken down, leaving a clear liquid that is easier to process (Wolff and hay, 1991).
Meulenberg and Hofman (1990) and Lequin et al. (1986) demonstrated that sonification of saliva yielded significantly higher levels of most steroids than centrifugation of saliva. It was suggested that sonificating the saliva samples disperses some unknown substance(s) which interfere with the analysis. Another reason for the higher levels is that after centrifugation one loses drug bound to cell debris, particulate matter or mucoprotein. (Anavekar et al., 1978).
On the other hand, in forensic work in which saliva samples have been taken primarily for serological purposes, it is common practice to subject the sample and container to boiling water temperatures for 15-30 minutes prior to freezing. Only in cases in which the toxic material present in saliva is volatile or heat-unstable would this treatment be expected to be deleterious to later analysis of such saliva samples. Another point where opinions differ about is the difference in the point of time in which the pH of the saliva samples should be measured, i.e. either immediately after collecting the sample, or before analysis of the sample.
Probably when the researchers measure pH immediately after collection they use the pH to clarify the transport mechanism; however when pH is measured after thawing, the pH is used for the analytical procedure.
|
| Immunological methods |
Immunological methods of analysis have been widely used for monitoring drugs in saliva and other body fluids, mainly because of:
(i) their relative simplicity of use, requiring little or no extractive operations,
(ii) their application to large batch analyses,
(iii) and especially their sensitivity.
a) Value of radioimmuoassay (RIA) techniques
In 1978, the group from the Tenovus institute rekindled interest in the determination of hormones in saliva by applying radioimmunoassay (RIA) techniques. Their studies have been followed by a growing number of publications demonstrating the value of RIA methods, especially for the analysis of hormones in saliva, such as estradiol, progesterone, testosterone, cortisol, and cortisone. Drugs have also been measured with RIA including:
(i) Cocaine (cone and Weddington, 1989; Inaba, Stewart and Kalow, 1978),
(ii) Cannabinoids (gross et al., 1985),
(iii) Haloperidol (Yamazumi and Miura, 1981),
(iv) Theophylline (Mally, Keszei and Cserep, 1992),
(v) And cotinine (Benkirane, Nicolas, Galteau and Siest, 1991).
b) Non-radioactive immunological procedure
The enzyme multiplied immunoassay technique (EMIT®), is based on competitive protein binding using an enzyme as a label and an antibody as a specific binding protein.
The enzyme activity is related to the amount of drug in the sample and is measured spectrophotometrically. Although this assay is very easy to use and requires no radiochemical facilities, it is not used very often for monitoring saliva levels, sensitivity may be one major reason for this. However, some anticonvulsant drugs are measured in saliva with EMIT® including:
(i) Carbamazepine (Paxton and Donald, 1980), and phenytoin (Umstead, Morales and McKercher, 1986) had a lower limit of detection of 0.1 mg/ml with a coefficient of variation for the assay of <10%.
(ii) Theophylline (Goldsworthy, kemp and Warner, 1981; Siegel et al., 1990) was determined in saliva from asthmatic children. The lower limit of sensitivity of the assay was 0.8 mg/ml with a coefficient of variation for the assay of <5% (Siegel et al., 1990).
c) Additional assays
Boever et al. (1990) developed a chemiluminescence immunoassay (CIA) using isoluminol for the detection of estradiol in saliva. Furthermore, phenytoin was measured in 1 ml saliva samples by fluorescence polarization (FPIA) (Cai, Zhu and Chen, 1993).
|
| Thin-layer chromatography |
| Thin-layer chromatography (TLC) has the advantage of simplicity and allows the simultaneous determination of several samples. However the major limiting factor is the detection of the respective spots on the thin-layer plate at low drug levels in saliva. Therefore, this technique is not often used in the drug monitoring of saliva. |
| High-performance TLC (HPTLC) |
With the development of high-performance TLC (HPTLC), Drehsen and Rohdewald (1981) tried to reach a higher sensitivity and precision. They monitored salicylic acid, salicylamide, ethoxybenzamide, acetaminophen, and some other weak analgesics in saliva. They were able to detect between 5 and 50 ng of the respective drugs with relative standard deviations ranging from 1 to 6.8%. However, this procedure did not find wider application for saliva drug monitoring. Often, high performance liquid chromatography (HPLC) is used for the analysis of drugs in saliva.
HPLC with electrochemical detection was also used for the determination of delta-9- tetrahydrocannabinol (THC). (Thompson et al. 1987). In addition, mexiletine, an anti-arrhythmic drug was monitored in this way (Katagiri, Nagasako, Hayashibara and Iwamoto, 1991). HPLC was also used to optimize the separation of caffeine from its metabolites, theophylline and paraxanthine, in saliva (Moncrieff, 1991). Furthermore, quinine and analogues are among other drugs which have been routinely measured in saliva by with HPLC. Last but not least, Lam et al. (1993) developed a chlorhexidine assay that required only 200 ?l saliva sample. The detection limit of this HPLC assay is 50 ng/ml, which is a significant improvement compared to spectrophotometric detection. |
| Gas chromatography with mass spectrometry |
The most popular analytical procedure for the measurement at the nanogram or picogram level is based on gas chromatography (GC), or, in the hyphenated mode, with mass spectrometry (GC/MS). This assay was used to detect:
- Nitrazepam,
- Clobazam in the saliva of epileptic children,
- Cocaine, for 12 to 36 h after administration,
- Salivary testosterone in female subjects, Gould et al. (1986).
|
| Advantages of saliva v/s serum testing |
- Saliva reflects the biologically active (free) fraction of steroids in the bloodstream (unlike blood or urine which measures total levels).
- Collection time is more controlled which is critical for baseline testing of hormones with diurnal variation and assessment of hormone replacement therapy dosing.
- Non-invasive, simple, safe, stress free and painless.
- Private and convenient for patient and physician.
- Allows for multiple collections outside of hospital setting.
- Hormones are stable in saliva at room temperature for at least 3 weeks.
- Transport of saliva to laboratory by regular mail.
- Less expensive than blood testing.
- Non traumatizing.
- Avoids false negative biopsies.
- Relatively cheap, i.e. Cost-effective.
- Easy to carry.
Saliva as a mirror of the body
Testing of saliva is very important for diagnosis, essentially by reflecting tissue fluid levels of natural substances, tissue fluid levels of some drugs, hormonal status, immunological status, neurological status, and emotional status.
Saliva tests are used to detect HIV (FDA approved in 1995),
Saliva tests in forensic medicine,
Saliva tests in endocrinology /fertility,
Saliva tests for diabetes and other pancreatic disorders, chronic pancreatitis,…
Saliva tests in immunology:
for Immunoglobulins’ titers in general, to herpes simplex, and anti-gliadin antibodies in Celiac Disease.
Saliva tests in dentistry – stomatology,
Preventive – curative dental care, Caries risk subjects – microbiological tests, Periodontitis, gingival inflammation.
Saliva tests in OBS/GYN, e.g. prenatal sex determination via GNB saliva test.
Saliva tests for drug monitoring, e.g. Digoxin, gentamycin, and others.
Saliva tests for rapid screening of ethanol in saliva (1995), and drugs in saliva:
Phenobarbital, Cimetidine, Marigwana, Cocain, Methodone, Opiate, Nicotine, and Phencyclidine.
Saliva tests are also good for infectious agents:
MMR (mumps, measles, and rubella), Helicobacter pylori, Hepatitis B surface antigen, Hepatitis A antibodies, and Hepatitis C on the horizon, coming up soon. |
| Hormone testing |
Hormone testing is approached either through Panels or Single
hormone testing through:
post-menopause Panel(s),
female Hormone Panel(s),
male Hormone Panel,
adrenal Stress Index,
thyroid Hormone Panels, or
salivary steroid hormone analysis. Monitoring plasma steroid levels is essential for the clinical assessment of a patient's endocrine function.
Free Fractions in saliva associate more faithfully with clinical symptoms than total serum hormone levels (which mostly reflect the bound fraction). (Fig. 3).
Some Salivary hormone tested:
Progesterone, estradiol, testosterone, cortisol, dehydro-epiandrostenedione (DHEA), Estriol, Androstenedione, FSH & LH, Thyroid hormones, and Melatonin, etc. |
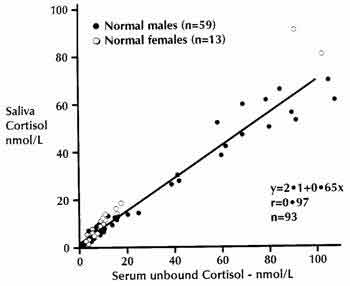
Figure 3: Correlation between Free Serum Cortisol Fraction and Salivary Cortisol. |
Conclusion
- Saliva is a useful diagnostic tool for the measurement of steroid hormones.
- Salivary concentration represents the free form of a particular hormone, and thus is a true reflection of its bioactivity.
- Moreover, the non-invasive nature of saliva collection and the convenience of multiple samples facilitate the design of functional assays for the assessment of various endocrine functions.
- In a recent review, Mandel has likened saliva to a mirror reflecting the emotional, hormonal, immuno-logical as well as nutritional and metabolic status of the body.
- The broad spectrum of interactions and relationships among these factors opens a whole field of diagnostic possibilities worth exploring and evaluating.
- Saliva test is a complete process from prescription, to collection, to results interpretation, diagnosis, and even to treatment and its monitoring.
|
| References |
1-Malamud D.
Saliva as a diagnostic fluid (editorial).
Br. Med. J., 305: 207-208, 1992.
2-Mandel I.D.
The diagnostic uses of saliva.
J. Oral Pathol., 19: 119-125, 1990.
3-Ferguson D.B.
Ed. Frontiers of Oral Physiol.
Karger, Basel., 5: 1-162, 1984.
4-Leucken L.J.
Childhood attachment and loss experiences affect adult cardiovascular and cortisol function.
Psychosom Med., 60 (6): 765-772, 1998.
5-Nashikawa Y; Jun L; Futai Y; Yanaihara N., Iguchi K; Mochizuki T; Hoshino M. and Yanaihara C.
Biomed. Res. 19(4): 245-251, 1998.
6-Freddy M.S.
Binding of the pili of pseudomonas aeruginosa to a low-molecular-weight mucin and neutral cystatin of human submandibular-sublingaul saliva.
Curr. Microbiol., 37(6): 395-402, 1998.
7-Jackson M. and Dudley D.J.
Endocrine assays to predict preterm delivery.
Clinics In Perinatology, 25(4): 834-857, 1998.
8-Raff H; Raff J.L. and Findling J.W.
Late-night salivary cortisol as a screening test for Cushing's syndrome.
J. Clin. Endocrinol and Metab., 83(8): 2681-2686, 1998.
9-Kudielka B.M; Hellhammer J; Hellhammer D.H; Wolf O.T; Pirke K.M; Varadi E; Pilz J. and Kirschbaum C.
Sex differences in endocrine and psychological responses to psychosocial stress in healthy elderly subjects and the impact of 2-week dehydroepiandrosterone treatment.
J. Clin. Endocrinol and Metab; 83(5): 1756-1761, 1998.
10-Mejicano G.C. and Maki D.G.
Infections acquired during cardiopulmonary resuscitation: Estimating the risk and defining strategies for prevention.
Ann. Intern. Med; 129(10): 813-828, 1998.
11-Dressendorfer R.A; Straburger C.J; Bidlingmaier F; Klug I; Kistner A; Siebler T. and Kiess W.
Development of a highly sensitive nonisotopic immuno-assay for the determination of salivary 17-hydroxyprogesterone: Reference ranges throughout childhood and adolescence.
Pediatr Res; 44(5): 650-655, 1998.
12-Embil J.M; Choudhri S.H; Smart G; Aldor T; Pettigrew N.M; Grahame G.R; Dawood M.R. and Bernstein C.N.
Comparison of salivary and serum enzyme immunoassays for the diagnosis of Helicobacter pylori infection.
Can. J. Infect. Dis., 9(5): 277-280, 1998.
13-Kennaway D.J. and Voultsios A.
Circadian rhythm of the free melatonin in human plasma.
J. Clin. Endocrinol and Metab. 83(3): 1013-1015, 1998.
14-Mandel I.D.
Salivary diagnosis: promises, promises.
Ann. N.Y. Acad. Sci; 694: 1-10., 1993.
15-Castle D. and Castle A.
Intracellular transport and secretion of salivary proteins.
Crit. Rev. Oral Biol. Med; 9(1): 4-22, 1998.
16-Baum B.
Principles of saliva secretion.
New York Acad. Sci; 694: 17-23, 1993.
17-Quissell D.
Steroid hormone analysis in human saliva.
New York Acad. Sci., 694: 143-145, 1993.
18-Frisch R.E.
Body fat, menarche, fitness and fertility.
Hum. Reprod., 2(6): 521-533, 1987.
19-Meulenberg P.M. and Hofman J.A.
Salivary progesterone excellently reflects free and total progesterone in plasma during pregnancy.
Clin. Chem., 35(1): 168-172, 1989.
20-Vuorento T; Lahti A; Hovatta O. and Huhtaniemi I.
Daily measurements of salivary progesterone reveal a high rate of an ovulation in healthy students.
Scand. J. Clin. Lab. Invest., 49: 395-401, 1989.
21-Walker S.M, Walker R.F. and Riad-Fahmy D.
Longitudinal studies of luteal function by salivary progesterone determination.
Horm. Res., 20: 231-240, 1984.
22-Connor M.L; Sanford L.M. and Lowland B.E.
Saliva progesterone throughout the menstrual cycle and late pregnancy.
Can. J. Physiol. Pharmacol., 60: 410-413, 1982.
23-Wong Y.F; Mao K; Panesar N; Loong E.P.L; Chang A.M.Z. and Mi Z.J.
Salivary estradiol and progesterone during the normal ovulatory menstrual cycle in Chinese women.
Eur. J. Obstet. Gynecol. Reprod. Biol., 34: 129-135, 1990.
24-Evans J.J; Steward C.R. and Merrick A.Y.
Oestradiol in saliva during the menstrual cycle.
Br. J. Obstet. Gynaecol., 87: 624-626, 1980.
25-Berthonneau J; Tanguy G; Janssens Y, et al.
Salivary oestradiol in spontaneous and stimulated menstrual cycles.
Human Reprod., 4: 625-628, 1989.
26-Mounib N; Sultan C.H; Bringer J; Hedon B; Nicolas J.C; Cristol P. and Bressot N.
Descomps. Correlations between free plasma estradiol and estroyens determined by bioluminescence in saliva, plasma and urine during spontaneous and FSH stimulated cycles in women.
J. Steroid Biochem., 31: 861-865, 1988.
27-De-Boever J; Kohen F; Bouve J; Leyseele D. and Vanderkerckhove D.
Direct chemiluminescence immunoassay of estradiol in saliva.
Clin. Chem., 36: 2036-2041, 1990.
28-Smith R.G; Besch P.K; Dill B. and Buttram Jr. V.C.
Saliva as a matrix for measuring free androgens: comparison with serum androgens in polycystic ovarian disease.
Fertil. Steril., 31: 513, 1979.
29-Walker R.F; Wilson D.W; Read G.F. Riad-Fahmy D.
Assessment of testicular function by radioimmunoassay of testosterone in saliva.
Int. J. Androl., 3: 105, 1980.
30-Gaskell S.J; Pike A.W. and Griffiths K.
Analysis of testosterone and dehydroepiandrosterone in saliva by gas chromotographymass spectrometry.
Steroids, 36: 219-228, 1980.
31-Gould V.J; Turkes A.O. and Gaskell S.J.
Gas chromatography-mass spectrometric analysis of salivary testosterone with reference to diethylstiboestrol-treated with prostatic cancer patients.
J. Steroid Biochem. 24: 563-567, 1986.
32-Read G.F; Harper M.E; Peeling W.B. and Griffiths K.
Changes in male salivary testos-terone concentration with age.
Int. J. Androl., 4: 623-627, 1981.
33-Cook N.J; Read G.F; Walker R.F; Harris B. and Riad-Fahmy D.
Changes in adrenal and testicular activity monitored by salivary sampling in males throughout marathon runs.
Eur. J. Appl. Physiol., 55: 634-638, 1986.
34-Vining R.F; McGinley R.A; Maksvytis J.J. and Ho K.Y.
Salivary cortisol: a better measure of adrenal cortical function than serum cortisol.
Ann. Clin. Biochem., 20: 329-335, 1983.
35-Laudat M.H; Cerdas S; Fournier C. and Giuban D.
Salivary cortisol measurements: a practical approach to assess pituitary adrenal function.
J. Clin. Endo-crinol Metab., 66: 343-348, 1988.
36-Vining R.F; McGinley R.A. and Symons R.G.
Hormones in saliva: mode of entry and consequent implications for clinical interpretation.
Clin. Chem., 29: 1752-1756, 1983.
37-Umeda T; Hiramatsu R; Iwaoka T; Shimada T; Miura F. and Sato T.
Use of saliva for monitoring unbound free cortisol levels in serum.
Clin. Chem. Acta., 110: 245-253, 1981.
38-Peters J.R; Walker R.F; Riad-Fahmy D. and Hall R.
Salivary cortisol assays for assessing pituitary-adrenal reserve.
Clin. Endocrinol Oxp. 17: 583-592, 1982.
39-Kahn J.P; Rubinow D.R; Davis C.L; Kling M. and Post R.M.
Salivary cortisol: a practical method for evaluation of adrenal function.
Biol. Psychiatry., 23: 335-349, 1988.
40-Tunn S; Mollmann H; Barth J; Derendorf H. and Krieg M.
Simultaneous measurement of cortisol in serum and saliva after different forms of cortisol administration.
Clin. Chem., 38: 1491-1494, 1992.
41-O'Connor P.G. and Corrigan D.L.
Influence of short-term cycling on salivary cortisol levels.
Med. Sci. Sports Exercise, 19: 224-228, 1987.
42-Tarui H. and Nakamura A.
Saliva cortisol: a good indicator for acceleration stress.
Aviat. Space. Environ. Med., 58: 573-575, 1987.
43-Guechot J; Lepine J.P; Cohen C; Fiet J. and Lemperiere T.
Simple laboratory test of neuroendocrine disturbance in depression: 11 p.m. saliva cortisol.
Neuro-psychobiology, 18: 1-4, 1987.
44-Robinson J; Walker S; Read G.F. and Riad-Fahmy D.
Salivary eostriol in normal pregnancy.
Lancet, 1(8229): 1111-1112, 1981.
45-Fischer-Rasmussen W; Gabrielsen M.V. and Wisborg T.
Relation of estriol in saliva to serum estriol during normal pregnancy.
Acta. Obstet. Gynecol-Scand., 60: 417-420, 1981.
|
| |
| المجلد 3 , العدد 4 , جمادى الأولى 1425 - تموز (يوليو) 2004 |
|
|
|
