| المجلد 4 ,
العدد 8
, ذو الحجة 1428 - كانون الثاني (يناير) 2008 |
| |
| Hyperparathyroidism and Hypothyroidism in Individuals Consuming High Fluoride Intake in Jeddah-Saudi Arabia |
| فرط الدريقات وقصورها لدى أفراد يتناولون جرعات عالية
من الفلوريد في جدة في المملكة العربية السعودية
|
| Suhad Maatoug Bahijri*, Abdullah Al-Fares*, Tariq Al-Khateeb** and Asaad M.B. Mufti*** |
| سهاد معتوق بهيجري، عبد الله الفارس، طارق الخطيب و أسعد مفتي |
| *Faculty of Medicine and Allied Sciences, **Faculty of Dentistry, ***Faculty of Earth Sciences, King Abdulaziz University, Jeddah, Saudi Arabia |
| Introduction |
An inverse relation between caries and water borne fluoride ranging from 0-2 ppm was found by Dean (1), and was confirmed later by Weaver (2). The optimum dose in drinking water to reduce caries incidence is accepted as ≈ 1 ppm in temperate climate (3) and lower (0.6 mg) in hot climate (4). Higher levels are associated with mottling (5) and increased incidence of caries (6). Moreover, non-skeletal effects of excess fluoride were noted in animals, and humans. These effects included reduced thryroxine and triiodothyronine (7), reduced blood calcium (8) and increased serum PTH concentration (9). Over the past 50 years, especially with the wide spread use of fluoride in dental products, the availability of fluoride and consequently exposure of the population to it has increased significantly (10-12). The situation is made critical for children by the inappropriate use of daily fluoride supplements. The situation is not much different in the adult population if tea is drunk excessively, and fluoridated toothpaste and mouth rinses are used liberally. The net result is that the population may be consuming more fluoride than the 1 mg/day dose suggested to produce maximal dental caries reduction with minimal risk.
High incidence of fluorosis were reported in different parts of the kingdom (13-16). This drew the attention to the possibility of increased intake of fluoride amongst Saudi population.
|
| Aim |
|
To carry out a pilot study on inhabitants of the Jeddah area to investigate the various sources and amounts of fluoride ingested, in order to find out whether there is a risk of fluoride toxicity or not, and to see whether excessive intake, if present, has caused adverse effects on the thyroid and parathyroid function of the selected individuals.
|
| Materials and Methods |
A-Selection of Subjects and Collection of Samples and Dietary Data
Healthy subjects were recruited for the study from attendants of the screening clinics at the Faculty of Dentistry as well as from the student population at King Abdulaziz University, in the following groups:
i-7 - 12 years - 30 subjects
ii-13 - < 20 years - 30 subjects
iii-20 - 50 years - 85 subjects
Principals of random selection were used in selecting subjects, and individuals not willing to take part in the study or those discovered to have genetic, internal, or metabolic disorders prior to selection were excluded from the study. Dietary interviews were conducted with all participants using a specially designed questionnaire that included information on their water and other fluids and food intake during the past 24-hours. The subjects were then given an appointment for dental examination (if not already performed at the screening clinic), and blood analysis. At the same time they were given a seven day food frequency questionnaire specially designed to include foods and beverages most likely consumed and of known fluoride content, and asked to return it on the day of the appointment. Compiling information from the two forms was used in calculating daily fluoride intake. First morning urine, and a sample of their drinking water was also requested.
On the day of the given appointment, blood samples were collected from fasting subjects, for the estimation of Parathyroid hormone (PTH), free triiodothyronine (FT3), free thyroxine (FT4), Thyroid stimulating hormone (TSH), calcium, phosphate, and albumin. Dental examination was carried out also.
The subjects’ weight (to the nearest 0.5 kg), and height (to the nearest 0.5 cm) were measured and the body mass index (BMI) was calculated.
All collected urine and sera samples were frozen at -20°C for analysis at the end of collection time.
B-Dental Examination
A collaborating dentist examined the teeth of all recruited subjects to determine the presence or absence of caries without giving a DMFT score. They were also examined for fluorosis using Dean’s criteria according to the WHO guidelines (17).
C- Calculation of Fluoride Intake
The minimum fluoride intake of all subjects was calculated by adding the calculated intake from water and beverages (analyzed in our laboratory) to that of the types of food included in the 24-hour recalls and 7 days food frequency questionnaire. Fluoride content of foods was obtained from various published sources (18-22) and when a range was quoted, the midpoint figure was taken. For children admitting to swallowing toothpaste or mouth-rinses, 25% of the fluoride content of the amount used of these products was added to the total from food and beverages(23). The average amount of toothpaste used was based also on earlier studies (24). As for children not admitting to swallowing toothpaste and brushing twice a day an average of 0.2 mg/day was used based on study by U.S. Department of Health and Human Resources (25). Half of that amount is used for children brushing once/day and 3/2 of the amount is used for those brushing 3 times/day.
It was difficult to estimate an amount of fluoride ingested when using mouth-rinse. However the same report mentioned earlier (25) gave an estimate for adults using daily mouthwash. Taking into account the difference in quantity of mouth-rinse used by a child compared to an adult, half of the amount estimated to be ingested by the adult is used when calculating the daily intake of children. The amount was multiplied by 3/7 when the child used the mouth-rinse 3 times/week, or by ½ if he used it every other day. Amounts ingested by adults when using tooth brush 2 times/day, or mouth-wash once a day were taken from the same report (25), and added to the intake from food and beverages. Adjustment to varying practices in teeth brushing and use of mouth-rinse were made similar to what was described earlier for children.
D- Biochemical Estimations
Albumin, calcium, and phosphorus
were estimated in serum samples using Hitachi 917 clinical chemistry analyzer and special reagent packs by Roche, in the biochemistry laboratory at the university hospital. Free triiodothyronine (FT3), free thyroxine (FT4), thyrotropin (TSH), and parathyroid hormone (PTH) were estimated using an automated electro-chem-illuminescent immunoassay “ECLIA” utilizing special reagent packs (Roche) and the Roche Elecsys 2010 immunu-assay analyzer.
Fluoride was determined in water, urine and various beverages using potentiometric measurements, made with a fluoride-ion specific electrode (Orion model 96-09 combination electrode) connected to ion analyzer (Orion model 720 A) using standard methods (26).
E-Statistical Analysis
The mean and SD of all biochemical parameters were calculated for each subgroup in the three age groups according to the fluoride intake. Means of the subgroups with high intake were compared to means of corresponding groups with low or optimal intake using unpaired student's t-test with significance being assigned at p<0.05.
|
| Results |
1-Analysis of fluoride in water and beverages
35 samples were analyzed. Fluoride concentration in tap water was similar to that in water sold as gallons (range 0.02 to 0.028 mg/l). Fluoride concentration in bottled water ranged from 0.045 to 1.06 mg/l, with 21 types having a concentration >0.5 mg/l, 2 a concentration of >0.25 mg/l, and 2 a concentration of <0.1 mg/l.
Fluoride concentration was estimated in 9 different brands of tea prepared in three different ways to obtain light, medium and dark brews. Infusions using two types of green tea were also analyzed. The concentration of fluoride in light tea ranged from 0.406-0.862 mg/l, while the range in medium brew was 0.89-2.56 mg/l, and 1.162-3.26 mg/l for dark brewed tea. Green tea had a concentration of 3.16 mg/l for one brand, and 4.08 mg/l for another brand.
The fluoride concentration in Arabic coffee ranged from 0.049-0.238 mg/l, while the range in Turkish coffee was 0.067 to 0.082 mg/l, and the range in instant coffee (4 brands) was 0.066 to 0.098 mg/l.
Fluoride concentration in soda drinks (4 brands including regular and light) ranged from 0.026 mg/l to 0.11 mg/l, while the range in energy drinks (4 brands) was 0.067-0.33 mg/l. The range in fruit flavoured drinks (14 types from 8 brands) was 0.045-0.123 mg/l, while that in fruit juices (14 types from 9 brands) was 0.094-0.506 mg/l.
2- Fluoride Intake of All Subjects
The fluoride intake of all age groups from different sources is presented in table 1. The number of subjects ingesting more than the recommended daily amount (27) are also shown. The highest percentage of subjects with high intake was found in the adult group, and the lowest in the teenage group. High intake was due to different causes according to age groups. Causes included excessive use of fluoride containing dental products, tea drinking, and ingestion of water containing >0.7 mg/l of fluoride. In the children and teenagers groups, no one cause was responsible for the high intake, but a combination of two or more, while in the adult group tea drinking and /or overzealous use of dental products were behind the high intake.
|
Table 1- Range of fluoride intake of all subjects from different sources (mg/day) and number of subjects exceeding the recommended intake
Different Sources
of Fluoride |
Fluoride Intake (mg/day) |
Children Groups n=30 |
Teenage Groups n=30 |
Adult Group |
No Fluorosis n=70 |
With Fluorosis n=15 |
Toothpaste |
0-0.438 |
0.01-0.225 |
0.01-0.225 |
0-0.225 |
Mouthwash |
0-0.25 |
0-0.5 |
0-0.5 |
0-0.5 |
Water |
0.02-1.267 |
0.02-1.267 |
0.02-1.478 |
0.02-1.478 |
Tea and Coffee |
0-1.02 |
0-1.272 |
0-1.7 |
0.02-2.975 |
Cold Beverages |
0.05-0.1 |
0.01-0.1 |
0.015-0.3 |
0.015-0.3 |
Food |
0.2-1.6 |
0.15-2.4 |
0.24-2.6 |
0.67-1.52 |
Total Intake |
0.546-3.284 |
0.86-5.1 |
0.97-5.7 |
1.13-5.85 |
No. of Subjects with Higher Than Recommended Intake |
16 |
6 |
46 |
9 |
|
3- Urinary Fluoride Levels in the Studied Groups
The results of estimating fluoride in urine samples from all subjects are presented in table 2. as mean ± SD and actual ranges of different sub groups. Our results are in the range of published data (26, 28-29). The means of the high intake sub groups were all significantly higher than the mean of the corresponding low or optimal intake sub groups. This validates our division of subjects into low or optimal and high intake sub groups, since urinary fluoride excretion is the recommended method of monitoring intake (30). However, there was an over lap between the values of the 2 sub groups in the children’s group and the group of adults without fluorosis. The over lap was mostly in the range of 0.41 - 0.64 mg/l.
4- Biochemical Evaluation of Study Groups
The mean ± SD of the different biochemical parameters and blood indices are calculated for the low or optimal fluoride intake and the high fluoride intake sub groups of the study groups. Results are presented in Table 3.
|
Table 2. Urinary Fluoride Levels in the Different Sub Groups.
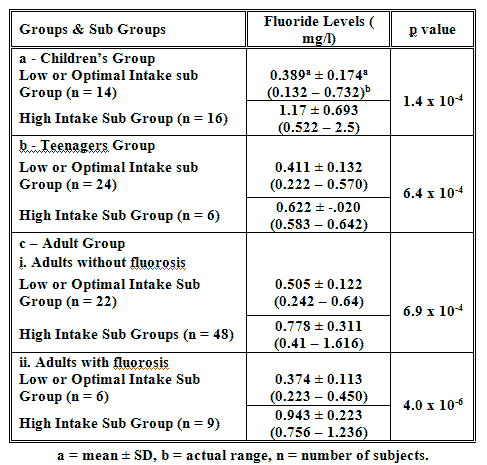 |
Mean TSH was significantly higher in the high intake sub groups of children, teenagers, and adults with fluorosis compared to the corresponding means of low or optimal intake sub groups. However no significant difference between the means of the sub groups of adults without fluorosis could be detected, but some subjects in the high intake sub group had higher than normal values.
- Mean FT3 and Mean FT4 were both significantly lower in the sub group of adults with fluorosis compared to the corresponding means of the sub groups of low or optimal intake. Moreover, some subjects in the high intake sub group of adults without fluorosis had lower than normal FT4.
- The means of PTH levels in all the high intake sub groups were significantly higher than the means of the corresponding low or optimal intake sub groups, and many subjects had above normal values (i.e. hyperparathyroidism).
- The means of phosphate values in all the high intake sub groups were significantly lower than the means of the corresponding low or optimal intake sub groups with some values below normal level.
- Mean calcium levels in the high intake sub groups of children and adults without fluorosis were both lower than the corresponding means of the low or optimal intake sub groups.
|
| Discussion |
A high percentage of our studied subjects were ingesting higher than recommended intake of fluoride daily. The calculation of the intake was validated by estimating fluoride in urine samples. The mean value in the high intake sub group was always significantly higher in all age groups (Table 2). The over lap in values noted in the children group and the group of adults without fluorosis could be due to various factors(31,32) including variations in urinary flow rate or pH, as well as differences in fluoride deposition/retention by the skeleton due to individual differences in bone metabolism, such as may occur during growth and development. In fact, the lowest values in the children’s high intake sub group were found in children with above average weight and height for age, indicating possible increased fluoride retention due to growth spurt. Similar findings were noted in earlier report (33), and similar suggestion was made. Higher than expected urinary levels in low or optimal intake sub group could be due to low ingestion of fluids generally, thus leading to more concentrated urine and higher urinary fluoride. However, since 24-hour urine sample was not collected, this cannot be verified.
The causes of high intake differed between different age groups, but tea drinking and over use of dental products singly or combined were the major causes in the adult population.
Fluorosis was only noted among adults, and it seemed to be due to excessive intake during early childhood as the subjects with fluorosis were all residents of rural areas during their childhood and depended on water from wells for drinking and cooking. This indicates that the danger of dental fluorosis is minimal in people residing in the Jeddah area all their lives. However, effects of fluoride excess on metabolism could not be ruled out. In fact, the results seen in Table 3 show clearly the adverse effects of excessive ingestion of fluoride.
Sub clinical hypothyroidism was noted in some subjects from all sub groups with high fluoride intake, indicated by high TSH levels and low or low normal FT4. However, this effect was not seen in all subjects. This was reported in earlier studies (8). The significantly higher mean TSH in the high intake sub group of adult subjects with fluorosis points to the reversible nature of the effect of fluoride on the thyroid, which was not reported in literature before.
In addition, hyperparathyroidism was noted in many subjects ingesting high amounts of fluoride in all study groups. This was seen in previous reports (9, 34-35). Results from the group of adults with fluorosis indicate the reversible nature of the effects of fluoride on the parathyroid. The high PTH levels have caused mean phosphate levels to be significantly lower in all sub groups with high fluoride intake compared to corresponding sub groups with low or optimal intake. However, it failed to increase mean calcium levels, and in fact, it was found to be significantly lower in the children group and the adult group without fluorosis.
Conclusion:
Higher than desirable fluoride levels are consumed by a large percentage of our population. This has adversely affected thyroid function in some subjects of all age groups leading to subclinical hypothyroidism. The parathyroid gland was also affected in some subjects with high intake leading to hyperparathyroidism, accompanied by hypophosphateamia when kidney function was not affected or hyperphosphataemia when kidney impairment was suspected. Hypocalcemia was also present in some cases. The effects of high fluoride intake on thyroid gland and parathyroid gland can be reversed if fluoride intake is decreased.
|
| References |
1. Dean H. et al. Domestic water and dental caries. V. Additional studies of the relation of fluoride domestic waters to dental caries experience in 4425 white children aged 12 to 14 years, of 13 cities in 4 states. Pub. Health Rep. 1942; 57:1155.
2. Weaver R. Fluorosis and dental carries on Tyneside. Brit. Dent J. 1944; 76:29 and 77:185.
3. Newbrun E. Effectiveness of water fluoridation. J. Public Health Dent. (Special issue) 1989; 279-289.
4. Lesan WR. Dental fluorosis: A review of literature with comments on tropical characteristics E. Afr. Med. J 1987; 64:493-497.
5. Dean GT. Et al. The investigation of physiological effects by the epidemiological method. In Moulton FR. Fluorine and Dental Health p.23. The Science Press Printing Company. Lancaster, Pennsylvania.
6. Ericsson SY. Cariostatic mechanisms of fluorides: Clinical observations. Caries Res. 1977; 11(supp.1.) :2.
7. Kendall-Tayler P. Comparison of the effects of various agents on thyroid adenyl cyclase activity with their effects on thyroid hormone release. J. Endocrinol 1972; 54:137.
8. Hillman D., Bolenbaugh DL. And Convey EM. Hypothyroidism and Anemia related to fluoride in Dairy Cattle. J. Dairy Sci. 1979; 62, 3:416-423.
9. Krishnamachari, KA. VR. And Sivakumar B. Endemic genu valgum. A new dimension to the fluorosis problem in India. Fluoride 1976; 9:185-200.
10. Fomon SJ., Ekstrand j., Ziegler EE. Fluoride intake and prevalence of dental fluorosis: trends in fluoride intake with special attention to infants. J. Public Health Dent. 2000; 60(3):131-139.
11. Pereira AC., Da CunhaFL., Meneghim M de C., Werner CW. Dental caries and fluorosis prevalence study in a nonfluoridated Brazilian community: trend analysis and tooth paste association. ASDCJ Dent. Child 2000; 67(2):132-5, 83.
12. Mascarenhas AK. Risk Factors for dental fluorosis: a review of the recent literature. Paediate. Dent. 2000; 22(4):269-277.
13. El-Tannir MD. Mottling of enamel in Mecca and the Arabian Peninsula. A survey and research study carried out in Saudi Arabia. Am. J. Public Health 1959; 49:45-52.
14. Akpata ES., Fakiha Z., Khan N. Dental fluorosis in 12-15 year old rural children exposed to fluorides from well drinking water in the Hail region of Saudi Arabia. Community Dent Oral Epidemiol 1997; 25(4):324-327.
15. Almas K., Shakir ZF., Afzal M. Prevalence, and severity of dental fluorosis in Al-Qaseem province Kingdom of Saudi Arabia. Odontostamatol Trop 1995; 22(85):44-47.
16. Al-Banyan RA., Echeverri EA., Narendran S., Keene HJ. Oral Health survey of 5-12 year old children of National Guard Employees in Riyadh Saudi Arabia. Paediat. Dent. 2000; 10(1):39-45.
17. World Health Organization. Oral Health Surveys – Basic Methods, 3rd edn. Geneva: WHO; 1987.
18. Scherz H. and Senser F. food Composition and Nutrition Tables 6th ed. Sonci SW., Fachmann W., Kraut H. ed. ISBN: 3-88763-076-9. Stuttgart, Germany. MED PHARM Scientific Publishers; 2000
19. Zohouri FV., Rugg-Gunn AJ. Fluoride concentration in Foods from Iran. Int. J. Food Sci. Nutr. 1999; 50(4):265-274.
20. Malde MK., Maage A., Macha E., Julshamn K., Bjorvatn K. Fluoride Content in Selected Food Items from Five areas in East Africa. J. Food Comp. & Anal. 1997; 10:233-245.
21. Anasuya A., Bapurao S., Paranjape PK. Fluoride and Silicon Content of Foods from Normal and Endemic Fluorotic Areas in India. J. Food Comp. & Anal. 1997; 10:43-48.
22. Dabeka RW., McKenzie AD. Survey of lead, cadmium, fluoride, nickel, and cobalt in food composites and estimation of dietary intakes of these elements by Canadians in 1986-1988. JAOAC Int. 1995; 78(4):897-909.
23. Whitford GM. Fluoride in dental products: safety consideration. J Dent Res 1987; 66:1056-1060.
24. Bentley EM., Ellwood RP., Davies RM. Fluoride ingestion from toothpaste by young children. Br. Dent. J. 1999; 186(9):460-462.
25. U.S. Department of Health and Human Resources, Review of Fluoride benefits and risks, Report of the Ad Hoc Subcommittee on Fluoride of the Committee to coordinate environmental health and related programs, Public Health Service, 1991; 8, 79, 81. Appendix D, 46 Table 27, 58, 87, 69.
26. Vandeputte M., DeCock J., Dryon L., Vercruysse A., Alexander F. and Massart DL. A contribution to the study of fluoride excretion. Clinica. Chemica. Acta 1977; 75:205-212.
27. Lewis DW., Limeback H. Comparison of recommended and actual mean intakes of fluoride by Canadians. JCDA 1996; 62(9):708-715.
28. Collins EM. and Segreto VA. Urinary fluoride levels of children residing in communities with naturally occurring fluorides in the drinking water. Journal of Dentistry for Children 1984; 51:352-355.
29. Marthaler TM., Steiner M., Menghini G., De Crousaz P. Urinary fluoride excretion in children with low fluoride intake or consuming fluoridated salt. Caries Research 1995; 29:26-34.
30. World Health Organization. Monitoring of renal fluoride excretion in community preventive programmes on oral health. Ed. Marthaler TM. Geneva: WHO, 1999.
31. Whitford GM & Pashley DH. The effect of body fluid pH on fluoride distribution, toxicity, and ranal clearance. In: Johansen E., Taves DR., Olsen T. eds. Continuing Evaluation of the Use of Fluorides, AAAS Selected Symposia 11. Boulder, CO: Westview Press, 1979:187-221.
32. Ekstrand J., Ehrnebo M., Whitford GM., Jamberg P. Fluoride pharmacokinetics during acid-base balance changes in man. European Journal of Clinical Pharmacology 1980; 18:189-194.
33. Ketley CE., Lennon MA. Urinary fluoride excretion in children drinking fluoridated school milk. Inter J Ped. Den. 2000; 10(4):260-268.
34. Srivastava RN., Gill DS., Mondgil A., Menon RK., Thomas M., Dandona P. Normal ionized calcium, parathyroid hyper secretion, and elevated osterocalcin in a family with fluorosis. Metabolism 1989; 38(2):120-124.
35. Gupta SK., Khan TI., Gupta RC., Gupta AB., Gupta KC., Jain P., Gupta A. Compensatory hyper parathyroidism following high fluoride ingestion – a clinico-biochemical correlation. Indian Pediat 2001; 38(2)139-146.
|
| |
| المجلد 4 ,
العدد 8
, ذو الحجة 1428 - كانون الثاني (يناير) 2008 |
|
|
|
