| المجلد 5 ,
العدد 10
, المحرم 1432 - كانون ثاني (يناير) 2011 |
| |
| Exhaled Nitric Oxide as an early marker of asthma |
| Fares A. Masri |
| Fares A. Masri
Faculty of pharmacy, University of kalamoon, Deratiah, Syria
|
| Review Article |
| Abstract |
According to the World Health Organization, asthma is now a serious public health problem with over 300 million sufferers. Despite the availability of effective therapies, asthma remains a source of significant morbidity and use of health care resources.
Measurement of fractional exhaled nitric oxide (FENO) has recently become of great interest in the diagnosis, treatment and monitoring of Asthma. Concentration of exhaled nitric oxide is markedly elevated in asthma, and its elevation is positively related to the degree of eosinophilic airway inflammation, airway hyperresponsiveness and symptoms.
Measurement of exhaled NO has been proposed as a noninvasive marker of asthma. Its measurement has recently been standardized by both American thoracic Society and European Respiratory Society, and there are now commercially available nitric oxide analyzers. This paper briefly reviews the role of Exhaled Nitric Oxide in the pathophysiology of asthma, and its role as a useful objective tool for diagnosing and monitoring asthma.
Keywords: Asthma, Exhaled Nitric Oxide, Inflammation, Measurement.
|
Abbreviations used in this paper:
NO, nitric oxide;
FENO, fractional exhaled nitric oxide;
NOS, nitric oxide synthase;
nNOS, neuronal nitric oxide synthase;
eNOS, endothelial nitric oxide synthase;
iNOS, inducible nitric oxide synthase;
NO2¯, nitrite;
NO3¯, nitrate;
RNS, reactive nitrogen species;
COPD, chronic obstructive pulmonary disease;
FEV1, forced expiratory volume in 1s.
|
| Nitric Oxide (NO): |
Nitric oxide is a free radical gas that is widely considered as one of the most important molecules in biology to have been studied in the past millennium. Recently, there has been a sudden increase of studies investigating the role of NO and its function in many diseases and conditions [1].
NO is a water- and lipid-soluble gas containing an unpaired electron, making NO a highly reactive molecule that participates in many chemical reactions and physiologic processes, among them vasodilatation, neurotransmission and host defense.
NO is derived from the amino acid,
L-arginine, via the enzyme, NO synthase (NOS) (Figure 1). At least three NOS isoforms, differing in activity and tissue distribution, have been identified. The constitutive NOS (eNOS and nNOS) localize mainly to endothelial and epithelial cells and to some of the airway nerves, whereas iNOS exists mainly in airway epithelial cells and inflammatory cells [2-4]. However, NO is also produced by many other types of cells, such as, macrophages, eosinophils, neutrophils, and neurons[5] that contribute to the roles of nitric oxide in respiration and as a bronchodilator [6, 7]. NO dilates human pulmonary arteries under normal pulmonary-circulation and respiratory conditions, whereas under hypoxic conditions, the release and action of nitric oxide are reduced [8].
In general, NO is highly reactive molecule with a short half-life (few seconds) in the bloodstream. As it is being produced, it is converted to stable end-products, such as nitrate, nitrite and peroxynitrite [1]. These stable end-products of NO can be measured in bronchoalveolar lavage or sputum, and their level was found to correlate with the level of exhaled NO [9]. The level of exhaled NO is the sum of the processes of synthesis, diffusion and metabolism that occur in the airway. It is increasingly proposed that high level of exhaled NO in asthma is due to the stimulation of iNOS in addition to the non-enzymatic NO production due to airway acidification [10].In short, nitric oxide is not merely a marker; it plays significant vascular and nonvascular regulatory and host-defense roles in pulmonary physiology and pathophysiology.
|
| No in the Lung: |
NO produced in the lungs is an important regulator of airway events, including modifying airway tone, regulating pulmonary vascular tone, stimulating mucin secretion, modulating mucociliary clearance through effects on ciliary beat frequency, immune surveillance including tumoricidal and bactericidal effects [4, 11-13].
The reaction of NO with other reactive species to produce reactive nitrogen species (RNS), including peroxynitrite (ONOO-) and nitrogen dioxide (NO2), is thought to be the prime reason why NO can contribute to the etiology of inflammatory lung disease [14, 15]. Despite extensive research into both pro-inflammatory and anti-inflammatory actions of NO, the overall contribution of NO to inflammatory conditions of the lung is not easily predicted and seems to depend on many factors, such as the site, time and degree of NO production in relation to the local redox status, and the acute or chronic nature of the immune response.
|
| Method for Measuring Exhaled Nitric Oxide: |
The first measurement of fractional exhaled nitric oxide (FENO) in normal human subjects [16] used chemiluminescence (based on a photochemical reaction between nitric oxide and ozone), diazotization, and mass spectrometry, and was later confirmed using gas chromatography /mass spectrometry [17]. FENO levels can be measured within a few minutes online during slow exhalation or offline from samples collected in bags. The former method is applicable to patients who are able to cooperate, the latter particularly useful in children under four years.
Measurement of FENO has been recently widely explored in asthma and has been correlated with predominantly eosinophilic airway inflammation [18-22] and to be diminished by corticosteroid therapy [23]. By using Nitric oxide analyzer, in which the flow rate of the exhaled air remains substantially constant, it is possible to detect any increase in the nitric oxide concentration of the exhales air and thus to conclude that there is inflammation in the lungs, but in which part of the lungs said inflammation is located cannot be determined by this method. Mathematical models on pulmonary NO dynamics have been published during the past few years , in which models the lungs are divided into two compartments, i.e. a bronchial compartment and an alveolar compartment [24, 25]. On the basis of these models it is possible to calculate separately bronchial NO flux and correspondingly alveolar NO concentration. On the basis of these parameters, it is possible to assess in which lung compartment according to the model the nitric oxide production has increased and/or the nitric oxide diffusion has changed, and hence it can be determined relatively reliably, in which lung compartment according the model inflammation may be located.
Some noticeable variations in exhaled–nitric-oxide values were reported. Most variations in exhaled nitric oxide can be explained by contamination from the upper respiratory tract [26]. Such contamination was not fully eliminated by the use of nasal blockage (encouraging only oral airflow). Rather, the problem was solved by the selection of an online single constant expiratory flow-controlled rate[27].
Even though the measurement of exhaled NO is highly accurate and extremely reproducible in normal and asthmatic subjects [28]; there are many methodological difficulties that should be considered. The European Respiratory Society and American thoracic Society published a set of recommendations for measuring exhaled and nasal NO in 2005 [29].Currently, several exhaled NO chemiluminescence analyzers are now commercially available as clinical tools (Figure 2).
|
| Role of Nitric Oxide in the Pathophysiology of Asthma: |
The role of NO in the pathogenesis of asthma has been initially observed in the early 1990s, when patients with asthma had high level of NO in exhaled air [19, 20]. With asthma recognized as an inflammatory disease, it has been suggested that elevated levels of NO in the exhaled breath of asthmatic patients may reflect ongoing inflammation[30]. Although several studies confirmed this finding [12, 19, 31-36], high levels of exhaled NO have been found also in other inflammatory lung diseases such as respiratory tract infections, fibrosing lung disease and rejection of a transplanted lung [37]. In patients with chronic obstructive pulmonary disease the exhaled NO level is considerably lower than in asthma; and most importantly, the major causative agent, tobacco smoking, dramatically reduces the level of exhaled NO and therefore masks any tendency towards a disease-related rise in exhaled NO levels [38].
Indeed, inhaled corticosteroids are reported to reduce exhaled [NO] in asthma patients due to their potent anti-inflammatory actions [39]. In addition, recent studies using steroid-naive patients with bronchial asthma have shown that exhaled [NO] is positively correlated with airway hyper-responsiveness and eosinophil count in induced sputum, further supporting the role of exhaled [NO] as a marker of asthma [40, 41].
Unfortunately, the exact mechanism by which NO contribute to the pathogenesis of Asthma is still poorly understood. We still do not know whether this high output of NO is defensive, destructive or simply just a marker of some other unknown mechanism in the pathogenesis of asthma. The NO that is formed in the airways may act as a bronchodilator and as an anti-inflammatory agent. However, in addition to these positive actions, NO may have a potential deleterious role in asthma, including increasing bronchial blood flow, exudation and edema, direct cytotoxic effect on airway epithelial cells. In vitro studies have suggested that the NO derived from airway epithelial cells may induce and amplify asthmatic inflammation by altering the balance between T helper type 1 and T helper type 2 cells, leading to an increase in the number of the latter and producing a cytokine profile that has been associated with asthma [42]. However, there is also contradictory evidence that NO plays a role in the down-regulation of adaptive immune responses and in the inhibition of the components of inflammation, including leukocyte proliferation, activation, migration and adhesion, production of leukotriens and other pro- inflammatory mediators [42-44]. Precisely how NO is involved in the pathogenesis of Asthma is unclear and needs to be better characterized.
|
| FENO, Potential Tool for Diagnosing and Monitoring Asthma: |
In the absence of an objective test for diagnosing and monitoring asthma, a vigorous research effort has subsequently sought to determine the nature of the relationship between Fractional exhaled nitric oxide (FENO) and asthma and to determine the clinical utility of FENO measurements in asthmatics. Evidence indicates that the levels of FENO in Asthma patients have been shown excellent correlation with esinophilic airway inflammation as represented by blood, sputum, bronchoalveolar lavage (BAL) and mucosal esinophilia [45, 46]. It is considered a valid and reproducible noninvasive marker with a high discriminatory capacity and can be used with more than 90% specificity for the diagnosis of asthma in both adults and children [47-51].
In the area of monitoring asthma, FENO correlates with symptom frequency and bronchodilator use but not with FEV1. In patients with subclinical airway inflammation in whom the FENO values are elevated, early anti inflammatory treatment may prevent subsequent remodeling and progression of asthma [52].
FENO values rapidly decrease with the use of inhaled corticosteroids and anti leukotriene therapy but not with neodocromil or theophylline[53]. It can therefore be used to predict the response to, and titrate the dose of, steroids. Furthermore, it can be used as a marker of loss of control and identify lack of adherence to anti-inflammatory medications. Considering the simplicity of the measurements, especially with portable NO analyzers, FENO can be used cost effectively for screening of large populations. However, lower levels of NO in patients treated with inhaled corticosteroids (ICS) should be taken into consideration, as this may reduce the sensitivity of NO as a diagnostic tool. In contrary, normal ENO levels may differentiate patients with chronic no asthmatic cough.
Although the direct measurement of exhaled NO is carried out by a non-invasive test that can be performed with ease, there is some evidence to suggest that exhaled NO may not reflect total NO production in the airways. Thus, the indirect measure-ment of NO in asthma by measuring the stable end-products (NO2¯ and NO3¯) in induced sputum can serve as an additional useful tool [9].
|
| Conclusion |
Measuring exhaled nitric oxide in the breath is a very attractive approach to diagnose and monitor asthma because it is noninvasive and makes repeated sampling possible However, there are important issues about reproducibility, variability, and sensitivity that need to be addressed before this approach can be recommended as an outcome measurement. There is relatively little information about how FENO relate to other clinical outcomes, such as progression of the disease, severity of disease, clinical subtypes, or response to therapy. In the future, new assays may be useful in predicting disease progression, indicating disease instability, and in predicting response to current therapies and novel therapies, many of which are now in development.
In summary, fractional exhaled nitric oxide (FENO) may provide an alternative noninvasive marker of inflammation in asthma that is convenient, quick, inexpensive and useful objective tool for diagnosing and monitoring asthma.
|
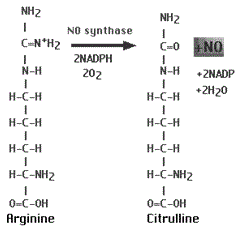
Figure 1: Nitric oxide production.
NO is synthesized from the conversion of L-arginine into L-citrulline by nitric oxide synthases in the presence of other co-factors including NADPH.


|
| References |
1. Moncada, S. and A. Higgs. The L-arginine-nitric oxide pathway. N Engl J Med, 1993. 329(27): p. 2002-p. 12.
2. Kobzik L, B.D., Lowenstein CJ, et al, Nitric oxide synthase in human and rat lung: immunocytochemical and histochemical localization. Am J Respir Cell Mol Biol, 1993(9): p. 371±7.
3. Asano K, C.C., Gaston B, et al, Constitutive and inducible nitric synthase gene expression, regulation, and activity in human lung epithelial cells. Proc Natl Acad Sci USA, 1994. 91: p. 10089±93.
4. Stuehr, D.J., Mammalian nitric oxide synthases. Biochem Biophys Acta, 1999. 1411(2-3): p. 217-230.
5. CJ, D.J., Snyder SH, Nitric oxide: a physiologic messenger. Ann Intern Med, 1994. 120: p. 227-237.
6. Adnot S, R.B., Eddahibi S, NO in the lung. Respir Physiol, 1995. 101: p. 109-120.
7. Di Maria GU, S.L., Mistretta A, Mazzarella G, Role of endogenous nitric oxide in asthma. Allergy, 2000. 55: p. 31-35.
8. D., M., Endothelium-derived relaxing factors and the human pulmonary circulation. Lung, 1990. 168: p. 35-42.
9. Kanazawa H, S.S., Yamada M, et al., Increased levels of nitric oxide derivatives in induced sputum in patients with asthma. J Allergy Clin Immunol, 1997. 99: p. 624-629.
10. Hunt JF, F.K., Malik R, et al., Endogenous airway acidification. Implications for asthma pathophysiology. Am J Respir Crit Care Med., 2000. (161) 694-699.
11. Dweik, R.A., et al., Nitric oxide synthesis in the lung. Regulation by oxygen through a kinetic mechanism. J Clin Invest, 1998. 101(3): p. 660-6.
12. Sanders, S.P., Nitric oxide in asthma. Pathogenic, therapeutic, or diagnostic? Am J Respir Cell Mol Biol, 1999. 21(2): p. 147-149.
13. Nathan, C. and Q.W. Xie, Nitric oxide synthases: roles, tolls, and controls. Cell, 1994. 78(6): p. 915-918.
14. Grisham, M.B., D. Jourd'Heuil, and D.A. Wink, Nitric oxide. I. Physiological chemistry of nitric oxide and its metabolites: implications in inflammation. Am J Physiol, 1999. 276(2 Pt 1): p. G315-21.
15. Beckman, J.S. and W.H. Koppenol, Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol, 1996. 271(5 Pt 1): p. C1424-1437.
16. Gustafsson LE, L.A., Persson MG, Wiklund NP, Moncada S., Endogenous nitric oxide is present in the exhaled air of rabbits, guinea pigs and humans. Biochem Biophys Res Commun, 1991. 181: p. 852-857.
17. Leone AM, G.L., Francis PL, Persson MG, Wiklund NP, Moncada S., Nitric oxide is present in exhaled breath in humans: direct GC-MS confirmation. Biochem Biophys Res Commun, 1994. 201: p. 883-887.
18. Alving K, W.E., Lundberg JM, Increased amount of nitric oxide in exhaled air of asthmatics. Eur Respir J, 1993. 6: p. 1368-1370.
19. Kharitonov, S.A., et al., Increased nitric oxide in exhaled air of asthmatic patients. Lancet, 1994. 343(8890): p. 133-135.
20. Gustafsson, L.E., et al., Endogenous nitric oxide is present in the exhaled air of rabbits, guinea pigs and humans. Biochem Biophys Res Commun, 1991. 181(2): p. 852-857.
21. Paiola G, T.L., Piacentini G, The measurement of exhaled nitric oxide in routine practice. Eur Ann Allergy Clin Immunol, 2009. 41: p. 131-135.
22. Bernstein JA, D.B., Alvarez-Puebla MJ, Nguyen D, Levin L, Olaguibel JM., Is exhaled nitric oxide a useful adjunctive test for assessing asthma? J Asthma, 2009. 46: p. 955-960.
23. Kharitonov SA, Y.D., Barnes PJ, Inhaled glucocorticoids decrease nitric oxide in exhaled air of asthmatic patients. Am J Respir Crit Care Med, 1996. 153: p. 454–457.
24. Lehtimaki L, K.H., Saarelainen S, Hahtola P, Jarvenpaa R, Koivula T, Turjanmaa V, Moilanen E., Extended exhled NO mesurment didrentates between alveolar and bronchial infalmaion. Am J Respir Crit Care Med, 2001. 163: p. 1557-1561.
25. Tsoukias N M, G.S.C., A two-compartment model of pulmonary nitric oxide exchang dynamics. J Appl Physiol, 1998. 85: p. 653-666.
26. Lundberg JO, W.E., Nordvall SL, Kuylenstierna R, Lundberg JM, Alving K., Primarily nasal origin of exhaled nitric oxide and absence in Kartagener's syndrome. Eur Respir J, 1994. 7: p. 1501-1504.
27. Silkoff PE, M.P., Slutsky AS, et al., Marked flow-dependence of exhaled nitric oxide using a new technique to exclude nasal nitric oxide. Am J Respir Crit Care Med, 1997. 155: p. 260-267.
28. Kharitonov SA, G.F., Kelly C, et al., Reproducibility of exhaled nitric oxide measurements in healthy and asthmatic adults and children. Eur Respir J, 2003. 21: p. 433–438.
29. Society, A.T., Recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide in adlts and children. Am J Respir Crit Care Med, 1999. 160: p. 2104-2117.
30. Djukanovic R, R.W., Wilson JW, et al., Mucosal inflammation in asthma. Am Rev Respir Dis, 1990. 142: p. 434–457.
31. Guo, F.H., et al., Molecular mechanisms of increased nitric oxide (NO) in asthma: evidence for transcriptional and post-translational regulation of NO synthesis. J Immunol, 2000. 164(11): p. 5970-5980.
32. Persson, M.G., et al., Single-breath nitric oxide measurements in asthmatic patients and smokers. Lancet, 1994. 343(8890): p. 146, 147.
33. Kharitonov, S.A., et al., Allergen-induced late asthmatic reactions are associated with elevation of exhaled nitric oxide. Am J Respir Crit Care Med, 1995. 151(6): p. 1894-1899.
34. Massaro, A.F., et al., Elevated nitric oxide concentrations in isolated lower airway gas of asthmatic subjects. Am J Respir Crit Care Med, 1996. 153(5): p. 1510-1514.
35. Silkoff, P.E., et al., Airway nitric oxide diffusion in asthma: Role in pulmonary function and bronchial responsiveness. Am J Respir Crit Care Med, 2000. 161(4 Pt 1): p. 1218-1228.
36. Mehta, S., et al., Acute and chronic effects of allergic airway inflammation on pulmonary nitric oxide production. Am J Physiol, 1997. 272(1 Pt 1): p. L124-131.
37. Lundberg JO, L.J., Alving K, Weitzberg E., Nitric oxide and inflammation: the answer is blowing in the wind. Nat Med, 1997. 3(1): p. 30, 31.
38. Sterk PJ, D.G.H., Ricciardolo FLM, Rame KF., Exhaled nitric oxide in COPD: glancing through a smoke screen. Thorax, 1999. 45: p. 565-567.
39. Kharitonov SA, Y.D., Barnes PJ, Inhaled glucocorticoids decrease nitric oxide in exhaled air of asthmatic patients. Am J Respir Crit Care Med, 1996. 153: p. 454-457.
40. Dupont LJ, R.F., Demedts MG, Verleden GM, Exhaled nitric oxide correlates with airway hyperresponsiveness in steroid-naive patients with mild asthma. Am J Respir Crit Care Med, 1998. 157: p. 894-898.
41. Jatakanon A, L.S., Kharitonov SA, Chung KF, Barnes PJ, Correlation between exhaled nitric oxide, sputum eosinophils, and methacholine responsiveness in patients with mild asthma. Thorax, 1998. 53: p. 91-95.
42. Taylor-Robinson AW, L.F., Severn A, et al., Regulation of the immune response by nitric oxide differentially produced by T helper type 1 and T helper type 2 cells. Eur J Immunol, 1994. 24: p. 980-984.
43. CD, M., Molecular basis of ``suppressor'' macrophages. Arginine metabolism via the nitric oxide synthetase pathway. J Immunol, 1991. 146: p. 2719-2723.
44. Wei XQ, C.I., Smith A, et al., Altered immune responses in mice lacking inducible nitric oxide synthase Nature. 1995. 375: p. 408±11.
45. Strunk RC, S.S., Phillips BR, Zeiger RS, Chinchilli VM, Larsen G, et al., Relationship of exhaled nitric oxide to clinical and inflammatory markers of persistent asthma in children. J Allergy Clin Immunol, 2003. 112: p. 883-892.
46. Lex C, F.F., Zacharasiewicz A, Nicholson AG, Haslam PL, Wilson NM, et al., Airway eosinophilia in children with severe asthma: Predictive values of noninvasive tests. Am J Respir Crit Care Med, 2006. 174: p. 1286-1291.
47. Dupont LJ, D.M., Verleden GM., Prospective evaluation of the validity of exhaled nitric oxide for the diagnosis of asthma. Chest, 2003. 123: p. 751–756.
48. JC, d.J., Surrogate markers of airway inflammation: inflammometry in paediatric respiratory medicine. Paediatr Respir Rev, 2000. 1: p. 354–360.
49. Barnes PJ, C.B., Kharitonov SA, et al., Pulmonary biomarkers in COPD. Am J Respir Crit Care Med, 2006. 174: p. 6–14.
50. Kharitonov SA, B.P., Exhaled markers of pulmonary disease. Am J Respir Crit Care Med, 2001. 163: p. 1693–1722.
51. Brodlie M, M.M., Exhaled nitric oxide in the diagnosis of childhood asthma. BMJ, 2009. 339: p. 5418.
52. Van den Toorn LM, O.S., de Jongste JC, Leman K, Hoogsteden SC, Prins JB., Airway inflammation is present during clinical remission of atopic asthma. Am J Respir Crit Care Med, 2001. 164: p. 2107-2113.
53. Lim KG, M.C., The use of fraction of exhaled nitric oxide in pulmonary practice. Chest, 2008. 133: p. 1232-1242.
|
| |
| |
| المجلد 5 ,
العدد 10
, المحرم 1432 - كانون ثاني (يناير) 2011 |
|
|
|
