| المجلد 6 ,
العدد 10
, شعبان 1434 - تموز (يوليو) 2013 |
| |
| ارتباط مستويات D-dimer البلازما مع شدة الإنتان والشفاء منه
لدى مرضى جراحة القلب في وَحْدَات الرِّعَايَةِ المُرَكَّزَة
|
| Plasma D-dimer Levels Correlate with Outcomes in Severely
Septic Cardio-Surgical Patients in the Intensive Care Units
|
| حسن محمد حالوش، عبد الرحمن غبره، مازن حالوش، عزمي الدرهلي، محمد حالوش، حسن سويد |
| Haloush M. H; Gabra A. R; Haloush Ma; Darhali A; Haloush Mo. And Swaid H. |
مستشفى تشرين ومستشفى أمية، دمشق، سورية.
Tishreen Hospital & Omayad Hospital
Damascus, Syria.
|
| الملخص Abstract |
الخلفية: يعد الإنتان الوخيم و الإنتان الدموي من الحالات المُمِيْتٌة ما بعد جراحة القلب. والغاية من هذه الدراسة هي تقييم القيمة الإنذارية لمستويات D-dimer لدى مرضى جراحة قلب مصابين بإنتان في وحدات الرعاية المركزة.
الطرق: أُجريت دراسة اسْتِباقِيّة قائمة على الملاحظة. واشتملت على مئة وسبع وعشرين مريضاً، أجريت لهم عمليات قلبية وأصيبوا بإنتان ممن عولجوا في وحدات الرعاية المركزة، في مستشفى تشرين ومستشفى أمية، في دمشق، سورية، في الفترة بين كانون الأول عام 2000 وكانون الأول عام 2009.
القياس: جرى قياس D-dimer البلازما باستخدام مقايسة لاتكس آلية. وسجلت عند قبول المريض في وحدة الرعاية المركزة وفي كل يوم حتى تخرّج المريض منها.
دُرست العلاقة بين D-dimer البلازما والمتغيرات الإنذارية في حَرَز (نمط) تقييم الصحة المزمنة II والفيزيولوجية الحادةacute physiology and chronic health evaluation II score (APACHE II) والحَرَز (النمط) II الفيزيولوجي التبسيطي الحاد simplify acute physiology score II (SAPSH)، وسجلت باستخدام التحليل ذي المتغير الوحيد وتحاليل التَحَوُّفٌ (التقهقر) الخَطِّيّ واللُّوجِسْتِيّ متعددة المتغيرات. وقورنت، على الترتيب، بين الباقين على قيد الحياة وغير الباقين على قيد الحياة.
النتائج: وجدت علاقة يُعتد بها بين مستويات D-dimer المرتفعة وAPACHE II و SAPS II. وكانت مستويات D-dimer المرتفعة مرتبطة بالإنتان الوخيم. وأظهرت قيمة D-dimer التنبؤية من أجل العلاج منطقة تحت المنحنى 0.78 (فاصلة موثوقية 95%، من 0.71 إلى 0.81)، لكن مستويات D-dimer كانت سلبية لدى 12% من مرضى الإنتان.
كانت القيمة الإنذارية لقيمة D-dimer البلازما كما يلي:
المرضى الذين فارقوا الحياة: كان متوسط مستويات D-dimer البلازمية أعلى من 4 مكغ/ مل.
المرضى الباقون على قيد الحياة: كانت قيمة D-dimer أقل من 2 مكغ/ مل (P< 0.0001).
الاستنتاج: يمكن أن تكون مستويات D-dimer البلازما مفيدة في التنبؤ بالنتائج السريرية لدى مرضى جراحة القلب المصابين بإنتان وخيم. وهي وسيلة مشابهة للـ APACHE II وSAPS II، فيمكن أن يكون D-dimer مساعداً في تَطَبُّق (تقييم درجة) الاختطار لدى مرضى الجراحة القلبية المصابين بإنتان.
|
| Plasma D-Dimer Levels Correlate with Outcomes in Severely Septic Cardio-Surgical Patients in The Intensive Care Units |
| Abstract |
Background: Severe sepsis and septicemias are lethal conditions following cardiac surgery. And the purpose of this study is to evaluate the prognostic value of plasma D-dimer levels in septic cardio-surgical patients in the intensive care units.
Methods: A prospective observational study was conducted, and it included one hundred twenty seven septic cardio-surgical patients treated in the intensive care units of tow hospital, Tishreen teaching hospital and Omayad hospital, in Damascus, Syria, from December 2000 to December 2009.
Measurement: Plasma D-dimer was measured using an automated latex assay and recorded on admission and every day until the patient left the ICU.
The relationships between plasma D-dimer and prognostic variables included in the acute physiology and chronic health evaluation II score (APACHE II) and simplify acute physiology score II (SAPSII) were examined, and recorded using univariate and multivariate linear and logistic regression analyses. And were compared respectively between survivors and non-survivors.
Results: A significant relationship was found between the presence of elevated D-dimer levels and the APACHE II and SAPS II.
Elevated D-dimer levels were associated with severe sepsis. And D-dimer predictive value for therapy showed an area under the curve of 0.78 (95% confidence interval, 0.71 to 0.81), but D-dimer levels were negative in 12% of the septic patients.
The prognostic value of plasma D-dimer levels was as following:
In non-survivors: the D-dimer plasma level mean value was higher than 4 mcg/ ml.
In survivors: the D-dimer value was less than 2 mcg/ ml (p < 0.0001).
Conclusion: The D-dimer plasma levels could be useful for predicting clinical outcome in cardiac surgical patients with severe sepsis. And it is similar to APACHE II and SAPS II, and D-dimer could be helpful in risk stratification in septic cardiac surgical patients.
|
| Introduction |
Patients after cardiac surgery have high incidence of infections, due to cardiac impairment, and intra-operative use of extracorporeal
circulation and its sequelae (1).
Accordingly, sepsis and infections, regardless of the focus of infectious are among the major causes of death, which vary from 17% to 65% (2-4).
The D-dimer results from the fibrin breakdown after fibrinolytic system activation, and its levels can be measured easily, and elevated levels of D-dimer have been detected in patients with, disseminated intravascular coagulation (DIC), severe sepsis and septic shock syndrome, thromboembolic events, including DVT and PE, liver disease, pregnancy, surgery, and Trauma (3, 4).
Several studies have demonstrated that elevated D-dimer levels on the admission of critically ill patients to the ICU is associated with an increased risk of mortality (5, 6).
Little is known about the relationship between D-dimer levels and the clinical outcomes of the septic cardio-surgical patients in the ICU.
Septic activation of the coagulation cascade is thought to lead to fibrin deposition in the intravascular mainly pulmonary vessels (7).
The main objective of our study was to evaluate outcome of cardiac surgery, that developed sepsis in the ICU period depending on the level of D-dimer.
|
| Materials and Methods |
From December, 2000, to December, 2009, in the Hospitals of Tishreen and Omayad, one hundred twenty seven septic cardio-surgical patients treated in ICU, with specific criteria for admission in the ICU.
Table 1: ICU Admission Criteria:
- Respiratory distress requiring oxygenation and ventilation support.
- Severe hypotension (systolic BP < 90 mm Hg) requiring inotropes.
- Oliguria with ongoing renal failure.
- Any mechanical support including, IABP, respirator, dialysis, etc…
- Deterioration of consciousness, from disorientation to coma.
- Uncontrolled coexisting disease, which affects the condition, e.g. CVA.
- Septic complications, or multiple organ failure syndrome.
Blood samples were prospectively obtained from those patients, who were older of sixteen years of age, and had received a diagnosis of Sepsis.
Table 2: Definition of sepsis, according to American College of Chest Physicians and Society of Critical Care Medicine consensus panel (8):
• Infection is a microbial phenomenon characterized by an inflammatory response to the presence of microorganisms.
• Bacteremia refers to the presence of viable bacteria in the blood.
• Systemic inflammatory response syndrome (SIRS) is clinically recognized by the presence of two or more of the following:
- Temperature >38°C or <36°C.
- Heart rate >90 beats/min.
- Respiratory rate >20 breaths/min, or PaCO2 <32 mmHg.
- WBC >12,000 cells/mm3, or <4000 cells/mm3, or >10 %.
• Sepsis is the systemic response to infection. Thus, it is SIRS+ infection.
• Severe sepsis is sepsis + organ dysfunction (hypotension, lactic acidosis, oliguria, or an acute alteration in mental status), with response to.
• Septic shock is sepsis with organ failure, means hypoperfused-hypotensive state, despite adequate fluid resuscitation, and the perfusion abnormalities are such as lactic acidosis, oliguria, or an acute alteration in mental status.
Blood was obtained from all patients, in the ICU, before starting antibiotic treatment, and patients were investigated using acute physiology and chronic health evaluation II score (APACHE II), table 3, and simplify acute physiology score II (SAPSII) (9,10).
Both scores were determined on the initial consultation of the patients, which consider sample of on-admission, then they were reviewed later on, and in the next 24 hours by one author (HMH), and it was repeated daily by the same one.
Patients were treated in the intensive care units, and the high dependency units by the implementation of sepsis guidelines of diagnosis and management.
|
Table 3: The APACHE II scoring system.
Variable |
High Abnormal Range Range Low Abnormal |
|
+4 |
+3 |
+2 |
+1 |
0 |
+1 |
+2 |
+3 |
+4 |
Temperature rectal (°C) |
>41° |
39- 40.9° |
|
38.5- 38.9° |
36 - 38.4° |
34 - 35.9° |
32 - 33.9° |
30 - 31.9° |
<29.9° |
Mean BP mm Hg |
>160 |
130-159 |
110 129 |
|
70- 109 |
|
50- 69 |
|
<49 |
Heart Rate (beat/min) |
>180 |
140- 179 |
110- 139 |
|
70- 109 |
|
55- 69 |
40- 54 |
<39 |
Resp. Rate
(RR/min) |
>50 |
35- 49 |
|
25- 34 |
12- 24 |
10- 11 |
6- 9 |
|
<5 |
O2 Index: PAO2- PaO2 (mm Hg) |
>500 |
350- 499 |
200- 349 |
|
<200 +
PO2>70 |
PO2 61-70 |
|
PO2 55-60 |
PO2<55 |
Arterial pH
Or (v) HCO3 |
≥7.7
>52 |
7.6-7.69
41-51.9 |
|
7.5-7.59
32-40.9 |
7.33-7.49
22-31.9 |
|
7.25-7.32
18-21.9 |
7.15-7.24
15-17.9 |
<7.15
<15 |
Serum Sodium (mEq/l) |
>180 |
160-179 |
155-159 |
150-154 |
130-149 |
|
120-129 |
111-119 |
<110 |
Potassium (mEq/l) |
>7 |
6-6.9 |
|
5.5-5.9 |
3.5-5.4 |
3-3.4 |
2.5-2.9 |
|
<2.5 |
Creatinine mg/dl |
>3.5 |
2-3.4 |
1.5-1.9 |
|
0.6-1.4 |
|
<0.6 |
|
|
Hematocrit
(%) |
>60 |
|
50-59.9 |
46-49.9 |
30-45.9 |
|
20-29.9 |
|
<20 |
WBC (/mm3) |
>40 |
|
20-39.9 |
15-19.9 |
3-14.9 |
|
1-2.9 |
|
<1 |
GCS: |
(15- actual GCS) |
A. Total Acute Physiology Score (sum of 12 above points) |
B. Age points (years) <44=0. 45 to 54=2. 55 to 64=3. 65 to 74=5. >75=6 |
C. Chronic Health Points: 5 = emergency surg, 2 = elective Surg. |
Total APACHE II Score (add together the points from A+B+C) |
Interpretation of Score:
Score |
0-4 |
5-9 |
10-14 |
15-19 |
20-24 |
25-29 |
30-34 |
<34 |
Death rate % |
4 |
8 |
15 |
25 |
40 |
55 |
75 |
85 |
|
| Study Protocol |
D-dimer was measured by the hospital laboratories, on the day that the specimen was collected. A value of more than 0.5 mcg/ml was considered to be a positive level.
Patients also underwent standard clinical and investigational evaluation that included medical history, physical examination, CXR, ECG, and general laboratories including (general tests, blood cell counts, C-reactive protein, ESR, prothrombin time, etc..).
Two blood samples for culture, urine culture, wounds oozing, and a sputum sample for Gram staining and culture were collected, before the antibiotic treatment was begun, when the patient could expectorate normally. A CXR was obtained on admission, and was repeated 48 hours later in all patients, and as needed.
Improvement was considered to be apparent when a clinical and investigational parameters performed 48h later showed an acceptable positive progression, and depending on APACHE II and SAPS II.
Where worsening was considered to have occurred when clinical and investigational parameters, and used scores became outside the normal limits.
All the patients admitted to the intensive care units were assessed twice daily, with a conclusion on the day of ICU-discharge, and hospital-discharge.
|
| Statistical Analysis |
Analytical approach regarding the statistics was determined to detect the relationships between patient outcomes and measurements of d dimer. Continuous variables were analyzed with the Student t test. Analysis of variance for normally distributed variables and the Kruskal-Wallis test for nonparametrically distributed variables were carried out. The Spearman rank correlation test was used for analysis of the linear relationship between D-dimer levels and quantitative variables.
The relationship between D-dimer and mortality was evaluated using a multivariate logistic regression analysis, in which D-dimer levels and APACHE II and SAPS II scores were considered to be independent variables. The area under the curve (AUC) of the receiver operating characteristic was used for the D-dimer mortality predictive value. All p values of < 0.05 were assumed to indicate statistical significance.
|
| Results |
During the study period, one hundred twenty seven patients were initially analyzed. Of these, eight patients were excluded from the study because they had a high probability of thrombo-embolic events, two patients due to previous hospital admission, in addition to fifteen patients because D-dimer measurements had been performed after starting antibiotic treatment. Thus, the final study cohort consisted of one hundred two patients, (127) – (8 + 2 + 15) = 102 patients.
There were no differences between patients included in the study and those excluded regarding age, sex, APACHE II and SAPS scores.
The ICU mortality rate was 22%, and the mean APACHE II score was 17.0 ± 4.
The D-dimer status correlated significantly with deterioration of general conditions, acquired supportive measures, altered mental disorders, acidemia, high glucose level, increasing creatinine, and positive reading of pulmonary consolidation.
The D-dimer levels showed an asymmetric distribution. The mean level was 2 mcg/ml; range from 0.5 to 8.0 mcg/ml. Its level was negative in 12% of the patients. At least 55% of the patients with D-dimer levels of > 1 mcg/ml, range between 0.75 and 2.25 mcg/ml. Although it was not very high, but there was a statistically significant correlation between the plasma D-dimer levels and APACHE II and SAPS II scores (r = 0.23; p < 0.001). The D-dimer levels were significantly higher in patients with hypotensive sepsis than in patients with normotensive sepsis (Table 4).
|
Table 4: D-dimer Levels and Clinical Course
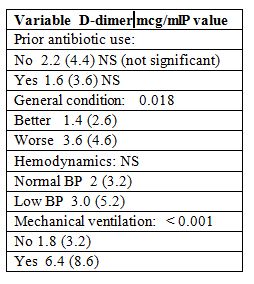
Table 5: The results of blood cultures

|
In addition, those patients who had a poor clinical course, also had higher D-dimer levels than those patients who showed improvement.
In fact, inotropic drugs including noradrenalin and mechanical ventilation were needed more frequently in patients with high D-dimer levels (Table 4).
The predictive value of D-dimer level for mechanical ventilation therapy showed an AUC of 0.78 (95% confidence interval, CI: 0.71 - 0.81).
The median (25th to 75th interquartile range) D-dimer levels observed in patients with major complications who died were more than 3 mcg/ml, in those with acute respiratory distress syndrome 3.3 mcg/ml, and in those with serious systemic decompensation of their baseline disease 3.6 mcg/ml.
A microbiological diagnosis was achieved in 48 of patients (34.3%), and the antibiogram was as following:
|
D-dimer levels strongly correlated with mortality: In no survivors, the mean D-dimer level was 3 ± 1.2 mcg/ml, while in survivors it was 1.5 ±1 mcg/ml (p < 0.0001).
For a cutoff level of 1 mcg/ml, the sensitivity for mortality was 97.4%, and the negative predictive value was 98.1%. In fact, only two patients died who had D-dimer levels of less than 1 mcg/ml, and D-dimer levels showed a high mortality predictive value with AUCs of 0.80 (95% CI, 0.75 to 0.84). The logistic regression model showed an independent relationship between D-dimer levels and mortality.
In addition, the mortality risk in patients with severe sepsis significantly increased when D-dimer levels were found to be > 3 mcg/ml (fig. 1).
|



|
| However, an AUC of the combination of both APACHE II and SAPS II, and D-dimer level of 0.92 (95% CI, 0.88 to 0.95) did not significantly increase the mortality predictive value (95% CI, 0.88 to 0.95) (fig 2). |
| Discussion |
This prospective study found that D-dimer status was often positive in patients with newly diagnosed sepsis, and it has demonstrated that ICU mortality was significantly greater among patients with high D-dimer levels.
The D-dimer levels correlated with well-established scoring systems for outcome prediction proposes such as APACHE II and SAPS II scores.
However, the relationship between D-dimer level and mortality was independent of these prognostic indicators, and even of others such as the type of surgery, the site of infection, the type of antibiotic administered, or the delay in its implementation.
Several investigators have addressed the relationship between D-dimer level and clinical outcomes in critically ill patients. Shorr et al (6) found that high D-dimer levels in critically ill patients were associated with increased in-hospital mortality, and also other studies (24, 25). Kollef et al (5)measured D-dimer levels in 123 patients who had been admitted to a medical ICU and demonstrated that increased plasma D-dimer concentrations were associated with clinical outcomes. Other authors have examined D-dimer levels in infectious diseases, in which histologic studies have demonstrated fibrin deposition in the pulmonary interstitium and alveoli.
More importantly, these authors found that D-dimer levels were associated with disease activity and levels of serum markers of inflammation (12).
It is well-known that D-dimer results from the breakdown of intravascular fibrin and can serve as a marker for fibrinolytic system activity. Additionally, growing evidence suggests that fibrin degradation products may enter into the circulation by the action of the fibrinolytic activity. Under inflammatory conditions, the hemostatic balance is shifted toward a predominance of procoagulant activity. In contrast, the fibrinolytic activity was found to be markedly reduced under these conditions (10, 11, 13, 14).
Multiple inflammatory cytokines may be involved in endothelial injury and dysregulation coagulation and fibrinolysis. Several authors (15,16) have demonstrated a relationship between markers of coagulation system activation, including D-dimer and cytokines such as interleukin 6. On the other hand, in the BAL fluids from patients with ARDS, the urokinase concentrations, representing the predominant plasminogen activator in this compartment, were markedly decreased, whereas elevated activities of plasminogen activator inhibitor-1 and alpha2-antiplasmin were consistently encountered (17). It has been demonstrated (18) that urokinase may induce selective fibrinolysis into the alveolar compartment, confirming the pathologic relevance of alveolar fibrin degradation. On the other hand, it has also been demonstrated (19) that inflammatory cytokines are capable of activating coagulation and inhibiting fibrinolysis in patients with severe sepsis (26, 27).
In the Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis study (3), which was performed in patients who had been hospitalized due to severe sepsis with systemic inflammation and organ failure due to acute infection, in 62% of whom the origin was pulmonary infection, the baseline plasma D-dimer and serum interleukin-6 levels were elevated, as was the expression of the inflammatory and procoagulant host response to infection.
In our study, which was performed in patients with sepsis criteria, we observed that D-dimer levels correlated with systemic fibrinolytic activation markers, such as leukocytosis, serum C-reactive protein, or bacteraemia, and other authors have observed similar results in their studies (20).
Our study has several limitations:
First, patients with positive D-dimer status were not systematically assessed to detect the presence of thromboembolic events, but a helical CT scanning, or compression ultrasonography were performed to rule it out only in those patients with a high pretest probability. Other authors have compared D-dimer levels between patients with sepsis and patients with a high probability of TEE, and found that patients with sepsis or TEE showed higher D-dimer levels that a control group, and they concluded that D-dimer measurement was useless in the differential diagnosis between sepsis and thromboemboism (21).
Second, a systematic study of the prothrombotic changes in the hemostatic system of patients in whom sepsis had been diagnosed was not performed. Prothrombotic changes in the hemostatic system associated with acute inflammation may provide the pathophysiologic link between infections and vascular disease. The measurement of fibrinogen, factor VII, prothrombin fragment 1 and 2, thrombin-antithrombin complexes, plasmin-antiplasmin complexes, tissue-type plasminogen activator, and plasminogen activator inhibitor-1 may help to establish the role of intravascular fibrinolytic activation in the increased D-dimer levels found in patients with sepsis.
|
| Summary |
Our study observed us, that D-dimer status is frequently positive in patients with Sepsis. And we found that D-dimer levels at the time of diagnosis of sepsis correlated with clinical outcome.
Acknowledgments
We thank our anesthetic, technician and nursing team, the Department of Cardiac Surgery, Tishreen Hospital and Omayad Hospital for their work and support.
We thank Dr. Catherine Moynihan , who is a consultant cardiac anesthetist and intensivist at west Birmingham hospitals in UK, for her manuscript review.
|
| |
1. Miholic J; Hudec M; Domanig E. et al.
Risk factors for severe bacterial infections after valve replacement and aortocoronary bypass operations.
Ann Thorac Surg; 40: 224-228, 1985.
2. Silva E; Fernandes JC; Akamine N. et al.
Sepsis and septic shock.
Editora Atheneu; 61-78, 2006.
3. Wada H; Sakuragawa N; Mori Y. et al.
Hemostatic molecular markers
before the onset of disseminated intravascular coagulation.
Am J Hematol., 1999; 60:273-278.
4. Bernard GR, Vincent JL, Laterre PF, et all Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med., 2001; 344:699-709.
5. Kollef MH, Eisenberg PR, Shannon W. A rapid assay for the detection of circulating D-dimer is associated with clinical outcomes among critically ill patients.
Crit Care Med., 1998; 26:1054-1060.
6. Shorr AF, Trotta RF, Alkins SA. et al.
D-direct assay predicts mortality in critically ill patients without disseminated intravascular or venous thromboembolic disease. Intensive Care Med; 1999, 25:207-210.
7. Abraham E. Coagulation abnormalities in acute lung injury and sepsis.
Am J Respir Cell Moi Biol; 2000, 22: 401-404.
8. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis.
Crit Care Med; 1992, 20(6): 864-874.
9. Knaus WA, Draper EA, Wagner DP. et al.
APACHE II: a severity of disease classification system.
Crit Care Med., 1985; 10: 818-829.
10. Idell S. Coagulation, fibrinolysis and fibrin deposition in acute lung injury.
Crit Care Med; 2003; 31 (suppl): S213-S220.
11. Gunther A, Mosavi P, Heinemann S. et al.
Alveolar fibrin formation caused by enhanced procoagulant and depressed fibrinolytic capacities in severe pneumonia.
Am J Respir Crit Care Med., 2000; 161:454-462.
12. Short AF, Hnatiuk OW. Circulating D dimer in patients with sarcoidosis.
Chest, 2000; 117:1012-1016.
13. Fuchs-Buder T, De Moerloose P, et al. Time course of procoagulant activity and D dimer in bronchoalveolar fluid of patients at risk for or with acute respiratory distress syndrome.
Am J Respir Crit Care Med., 1996; 153:163-167.
14. Abraham E.
Coagulation abnormalities in acute lung injury and sepsis.
Am J Respir Cell Moi Biol., 2000; 22:401-404.
15. Shorr AF, Thomas SJ, Alkins SA, et al.
D-dimer correlates with proinflammatory cytokine levels and outcomes in critically ill patients.
Chest, 2002, 121:1262-1268.
16. Pettila V, Hynninen M, Takkunen O, et al. Predictive value of procalcitonin and interleukin 6 in critically ill patients with suspected sepsis.
Intensive Care Med; 2002; 28:1220-1225.
17. Hasday JD, Bachwich PR, Lynch JP. et al.
Procoagulant and plasminogen activator activities of bronchoalveolar fluid in patients with pulmonary sarcoidosis.
Exp Lung Res; 1988; 14:261-278.
18. Ruppert C, Markart P, Schmidt R, et al.
Chemical cross-linking of urokinase to pulmonary surfactant protein B for targeting alveolarfibrin.
Thromb Haemost, 2002; 89:53-64.
19. Bone RC, Grodzin CG, Balk RA. Sepsis: a new hypothesis for pathogenesis on the disease process. Chest, 1997; 112:235-243.
20. Levi M, Schultz MJ, et al. Bronchoalveolar coagulation and fibrinolysis in endotoxemia and pneumonia.
Crit Care Med., 2003; 31(suppl): S238-S242.
21. Jimenez-Castro D, Perez-Rodriguez E, et al. Diagnostic value of D-dimer in pulmonary embolism and pneumonia.
Respiration, 2002; IV:229-233.
22. Wells PS, Ginsberg JS, Anderson DR, et al. Use of a clinical model for safe management of patients with suspected pulmonary embolism.
Ann Intern Med., 1998; 129:997-1005.
23. Fine MJ, Auble TE, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia.
N Engl J Med., 1997; 336:243-250.
24. Abdelnoor M, Nitter-Hauge S Trettli S. Relative survival of patients after valve replacement. Eur Heart J., 1990; 11:23-28.
25. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system.
Crit Care Med., 1985 Oct; 13(10): 818-829.
26. Ginsberg JS, Wells PS, Kearon C, et al. Sensitivity and specificity of a rapid whole-blood assay for D-dimer in the diagnosis of pulmonary embolism.
Ann Intern Med., 1998; 129:1006-1011.
27. American College of Chest Physicians.
Copyright, 2004 Gale Group.
|
| |
| المجلد 6 ,
العدد 10
, شعبان 1434 - تموز (يوليو) 2013 |
|
|
|
