| المجلد 6 ,
العدد 6
, شعبان 1433 - تموز (يوليو) 2012 |
| |
| Development of in House- Enzyme Linked Immunosorbant Assay (ELISA) for Sero-diagnosis of Salmonellae Infection in Patients from Damascus and Daraa Governorates |
| تطوير مقايسة الممتز المناعي المرتبط بالإنزيم (ELISA) في المُؤَسَّسَة من أجل تشخيص مصلي لعدوى السَّلْمونيلَة لدى مرضى من محافظتي دمشق ودرعا |
| د. عبد الغني العقاد و د. عصام صباغ |
| الجامعة العربية الدولية الخاصة، سورية |
| Al-Akkad Abd. Ghani and sabbagh I.
Arab International University (AIU), Syria
|
| الملخص Abstract |
Typhoid fever induced by Salmonella represents a serious problems, in many regions of the world, including Syria. Routine diagnosis of suspected typhoid cases is based on detecting agglutinating antibodies in patients sera. These methods are controversial due to lack of sensitivity and specificity. New methods like Enzyme linked Immunosorbant Assays (ELISA) for sero-diagnosis of Salmonellae infection have been developed. Here we describe development of a simple in house-ELISA format for detecting and epidemiological surveillance of typhoid suspected patient.
تمثل الحمى التيفية المُحْدَثة بالسَّلْمونيلَة مشكلة خطرة في العديد من مناطق العالم، بما فيها سورية. ويعتمد التشخيص الروتيني للحالات السريرية المشكوك بها على كشف الأضداد الراصة في مصول المرضى. وهذه الطرق مثيرة للجدل لافتقارها للنوعية والحساسية. ولقد جرى تطوير طرق جديدة للتشخيص المصلي لعدوى السَّلْمونيلَة مثل مقايسة الممتز المناعي المرتبط بالإنزيم (الاليزا). ونصف هنا تطوير طريقة إليزا مؤسساتية من أجل الكشف والترصد الوبائي للمريض المُشْتَبَه بإصايته بالتيفية.
|
| Introduction |
| Typhoid fever, a severe prolonged systematic infection by Salmonella typhi, S. paratyphi-A, S. paratyphi-B, and S. paratyphi-C, is an important cause of morbidity in many regions of the world1, with an estimated 22 million cases and 200,000 related deaths occurring worldwide each year2, and 13 million cases occurring annually in Asia alone3. Also, Infection with different strains of Salmonella is attributed to many cases of gastroenteritis (Salmonellosis), mediated by consumption of food and dairy products contaminated with these bacterial strains. Infection with systematic Salmonellae is endemic in different parts of Mediterranean countries including Syria, where 3841 confirmed typhoid fever cases are recorded by the national registry system of Syrian health authorities in 20094. These cases, in spite of decline in the last ten years, yet still represent a serious problem especially in rural areas of Syria. Emergence of resistant strains of Slmonella typhi, and S. paratyphi-A against traditional first-line drugs, fluoroquinolones, and now to third generation cephalosporins, is posing patient management challenges5. Lab diagnosis of systematic Salmonella is based on Bone-marrow culture as superior method in term of sensitivity6; 7, alter-natively blood culture as less invasive method, yet less sensitive, are routinely used8. Culture methods are time consuming, requiring specialized selective and differential culture media, which may be financially exhausting for health systems of developing countries. This explains the appreciation of retrospective sero-diagnosis of typhoid fever for epidemiological surveillance, and to confirm clinical suspected typhoid fever. Sero-diagnosis of Salmonella is based, traditionally, on defining agglutinating antibodies against somatic, and flagella Salmonella antigens in Widal test applied as a slide-, or tube-agglutination format. This test, while is offering a simple methodology, however, it has inherent poor sensitivity and specificity properties9, due to the polyvalent nature of the antigens involved, repeated exposures to S. typhi in endemic regions, cross-reactivities with other non-Salmonella organisms, lack of reproducibility of test result, and subsequently difficulty in establishing a steady-state baseline titer for the population10. All these factors forced towards developing more sensitive, and specific serologic methods for diagnosis of typhoid fever, and led to emergence of different format based enzyme linked immunosorbant assays (ELISA) in the last 30 years11- 17. These latter assays, while are replacing the slide-and tube-agglutination methods due to their superior sensitivity, specificity, and the high number of sample load to process in one single format, yet They are more expensive than Widal based assays. All these data promoted us to develop a rather simple, relatively cheap, yet reliable sensitive, in house ELISA assay format to confirm those suspected Syrian typhoid fever patients, and aid in conducting country-level in epidemiological surveillance |
| Materials: |
- S. typhi reference strain (ST); kindly provided by The United Nations Relief and Works Agency for Palestine Refugees in the Near East (UNRWA)
- Attenuated veterinary S. typhi
vaccine strain (S19); kindly provided by Dr. Husam Haj Ali; High institute for atomic energy
- 3, 3', 5, 5'-Tetramethylbenzydine (TMB) (sigma)
- Skimmed milk 5% in PBS (local provider)
- 96-well format microtiter plate (NUNC)
- (AHPIPA): Goat Anti-human polyvalent immunoglobulins (G, M, A)-peroxidase labeled antibody (sigma)
- Phosphate buffer saline (PBS) (NaCl 8gm, KCl 0.2gm, Na2HPO4 1.15gm, KH2PO4 0.2gm; PH=7.2)
- PBS work solution (tween20 in PBS; 0.5% v/v)
- Citrate Buffer (0.1M; PH=5.5)
- Tween 20; DMSO: Dimethyl Sulphoxide
- Formaldehyde 37%
- TMB stock solution: 5 mg TMB in 1 ml DMSO
- Color development substrate: TMB 250 µ l, 12ml Citrate buffer, 100 µl H2O2 3% v/v
- Color development stop solution: H2SO4 2N
- 96-well format ELISA microtiter plate reader (Sunstik®) at reading wave of 450 nm, reference reading wave of 630 nm
- Healthy apparently individuals derived Sera form Daraa, and Damascus governorates
- Serologically Lab confirmed typhoid fever patients derived Sera
- IFA (Investigation Pharmaceutical ALBO) manufactured febrile antigens, U.S.A
|
| Methods: |
1-Preparation of S.typhi derived antigens
Both Salmonella typhi reference strains (ST, S19) are inoculated on 500 ml trypticase soy bean broth, and grown for 16 hours at 37C°, followed by treating with either formaldehyde at final concentration of (0.5% v/v), or boiled for 30 min at 100 C°.
Killed S. typhi reference strains (STh,STf,S19h,S19f ; h and f denotes heat, and formaldehyde respectively) are centrifugated at 5000 RPM for 10 min, and bacterial pellets are washed by PBS work solution 3 times followed by adjusting to concentration of approximately 1.5X108 cells/ml.
2-Coating of 96-well format microtiter plates with Salmonella typhi antigens, and ELISA reading
100 µl of STh, STf, S19h, and S19f antigens are dispensed into 96-well format microtiter plates at concentration of 108 cells/ml and incubated for 24 hrs, at 4C°. Plates are washed 3 times with PBS work solution, and coated with skimmed milk 5% v/v in (PBS work solution without tween) for 1 h at 22 C°, followed by washing 3 times with PBS work solution.
Commercial falgellar and somatic Salmonella typhi derived antigens, used in performing Widal screening agglutination test, are used as positive control for assessing quality of in- house prepared antigens. 100µl diluted healthy apparently individuals derived Sera, or lab confirmed typhoid fever patients derived sera are dispensed into 96-well format ELISA microtiter plates, and incubated for 1 hour, followed by washing 3 times with PBS work solution. Then, 100µl of AHPIPA are dispensed into 96-well format ELISA microtiter plates, and incubated again for 1 hour, followed by washing 3 times with PBS work solution. Finally 100µl of color substrate solution is dispensed into all wells of Salmonella typhi derived antigens coated onto 96-well format ELISA microtiter plates for 15 min , then color development is stopped by adding 50µl color stop solution, followed by absorbance reading by ELISA reader at both λ = 450 nm, 630 nm respectively.
3-Titration of healthy and typhoid fever patients derived sera
Suspected typhoid fever patients sera and healthy individuals derived sera are tittered by slide screening agglutination method using IFA's febrile antigens, and titers of 1/80*, 1/160, and >1/160 are obtained for patients derived sera, and <1/80 for healthy derived sera respectively.
*Titer >=1/80 is considered as cutoff for positive, that to mean, confirmed lab result for typhoid fever suspected patient.
|
| Results |
1-Evaluation of different Salmonella typhi derived antigens for coating
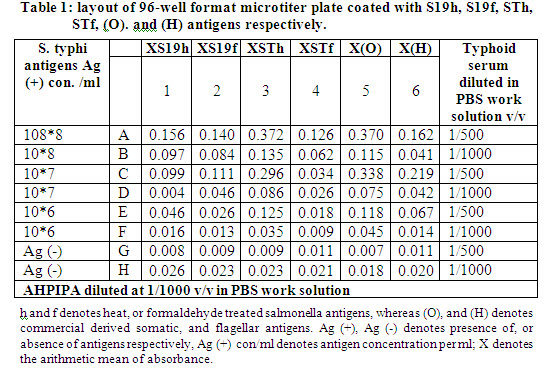
We aimed, at first, to define the suitable concentrations and convenience of both formaldehyde derived (STf, S19f), and heat treated Samlonella typhi derived antigens (STh, S19h) for coating onto 96-well format ELISA microtiter plates. Plated are coated with in house and commercial derived Salmonella typhoid antigens at 10fold serial dilution of antigens. Subsequently they are chess-board titrated with confirmed typhoid fever derived serum (agglutinating antibodies titer of 1/160) at dilutions of 1/500, and 1/ 1000 v/v in PBS work solution, followed by anti-human polyvalent immunoglobulins-peroxidase antibody (AHPIPA) diluted to 1/1000 v/v in PBS work solution (see methods). After color development, absorbances of microtiter plate’s wells are assayed. These experiment showed that the heat treated Salmonella typhi derived antigens (STh) have higher absorbance, and supposed better performance for the inteneded developed ELISA compared to formaldehyde treated Salmonella typhi derived antigens (STf), heat treated vaccine Salmonella typhi derived antigens (S19h), and formaldehyde treated vaccine Salmonella typhi derived antigens (S19f) respectively, when they are compared with commercial somatic antigens derived absorbances as a positive control, as shown in columns shaded in gray in table-1. Consequently heat treated Salmonella typhi derived antigens (STh) are chosen for coating in next experiments.
2-Titration of typhoid fever sera and (AHPIPA):
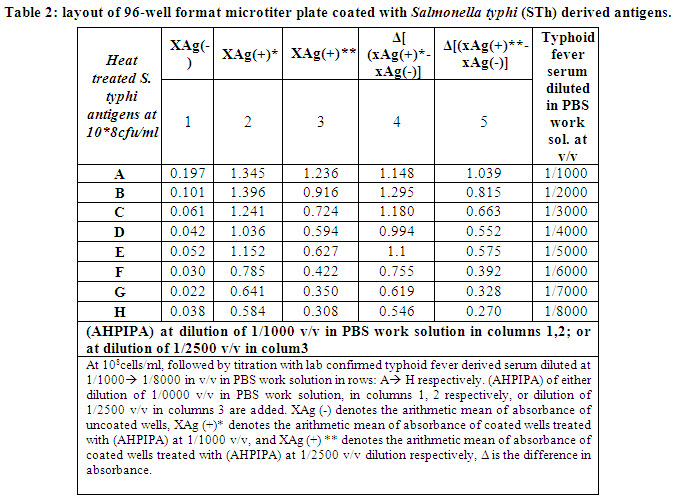
We aimed to define the best concentration of (AHPIPA) to be used in ELISA assay. For this purpose, a 96-well format ELISA microtitrer plate is coated with heat treated Salmonella typhi (STh) derived antigens at 108 cells/ml, followed by adding typhoid fever serum with titer of 1/160 agglutinating antibodies (defined as described in methods), at serial dilution of 1/1000; 1/2000; 1/3000; 1/4000; 1/5000; 1/6000; 1/7000; and 1/8000 v/v in PBS work solution. (AHPIPA) of either dilutions of 1/1000, or 1/2500 v/v in PBS work solution are added. This experiment showed that (AHPIPA) diluted at 1/2500 are suited for ELISA assay, as it still produce Δ absorbance ~10fold the absorbance of blank, at very high dilations range of 1/3000 -> 1/8000 as shown in wells shaded in gray in table-2.
3-Evaluation of sensitivity and specificity of developed ELISA assay
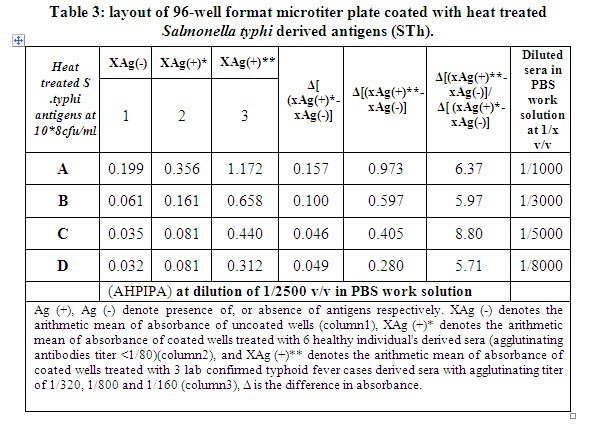
To evaluate the sensitivity of our developed EISA assay to detect suspected typhoid fever case, we aimed to test multiple apparently healthy individuals derived sera (tested to have agglutinating titer < 1/80), also lab confirmed typhoid fever derived sera with agglutinating titers of 1/80, 1/160, 1/320, and 1/800 respectively, at dilutions of 1/1000, 1/3000, 1/5000, and 1/8000 v/v in PBS work solution.
These experiments showed success of developed ELISA to detect, and discriminate typhoid fever cases, from apparently healthy individuals, where obtained absorbances for typhoid forever derived sera are > 5-6 fold of absorbances of healthy derived sera, as shown in columns shaded in gray in table-3.
|
|
| Discussion |
| Our data proved that a simple crude mixture of Salmonella typhi derived antigens coating ELISA format is adequate to discriminate typhoid fever suspected cases at acceptable level of sensitivity, compared to healthy individuals: 5-6 fold increase in absorbance, as we considered that 3 fold increase in absorbance of assayed suspected sera as cutoff for positive typhoid fever patient. This ELISA assay, though suffer from specificity, as no purified lipopolysaccharides (LPS)14;15, flagellar proteins18, outer membrane proteins (OMP) 13;19, or capsular polysaccharide Vi antigens are used11;20;21, nevertheless, compared to Widal assay, it is more suitable in term of sensitivity (sera diluted to 1/5000 -> 1/8000, are adequate to produce 5-6 fold increase in the measured absorbances compared to healthy individuals derived sera), also it has high sample load, and minimum sample volume, which makes it convenient to conduct cost saving epidemiological surveillance on Syria different governorate level, and replace Widal test still in practice. Our future research goal is to develop more optimized sandwich based anti-salmonella antibodies ELISA format using specific purified salmonella antigens, and capture monoclonal antibodies (mAb). |
| Acknowledgment: We express our deep thanks for Mr. Ahmad Abu-Ghayada at AIU, for his technical assistance. |
| References |
1-Pang T, Bhutta Z.A., Finlay B. B., and Altwegg M.
Typhoid fever and other salmonellosis: a continuing challenge.
Trends Microbiol. 3: 253-255. 1995.
2-Crump J.A; Luby S.P. and E.D. Mintz.
The global burden of typhoid fever.
Bull World Health Organ. 82: 346-353, 2004.
3-Ivanhof B.
The Third Asia Pacific Symposium on Typhoid Fever and Other Salmonellosis. S1-1, 1997.
4-Health statistical abstract.
5th issue, Table-31: number of communicable diseases, p. 108, 2009.
5-John A. Crump and Eric D. Mintz.
Global trends in typhoid and paratyphoid fever.
Clin. Infect. Dis. 15; 50(2): 241-246. 2010.
6-Farooqui B.J; M. Khurshid M.K. Ashfaq and M.A. Khan.
Comparative yield of Salmonella typhi from blood and bone marrow cultures in patients with fever of unknown origin.
J. Clin. Pathol. 44: 258-259, 1991.
7-Vallenas C; Hernandez H; Kay B; Black R. and Gotuzzo E.
Efficacy of bone marrow, blood, stools and duodenal content cultures for bacteriologic confirmation of typhoid fever in children.
Pediatr. Infect. Dis. 4: 496-498, 1985.
8-Wain J; Diep T.S; Ho V.A; Walsh A. M; Nguyen T.T; Parry C.M; and White N.J.
Quantization of bacteria in blood of typhoid fever patients and relationship between counts and clinical features, transmissibility,
and antibiotic resistance.
J. Clin. Microbiol. 36:1683-1687, 1998.
9-House D, Wain J, Ho VA, Diep TS, Chinh NT, Bay PV, Vinh H, Duc M, Parry CM, Dougan G, White NJ, Hien TT. and Farrar JJ.
Serology of typhoid fever in an area of endemicity and its relevance to diagnosis.
J Clin Microbiol. 39(3): 1002-1007, 2001.
10-Lateef A. Olopoenia and Aprileona L. King
Widal agglutination test -100 years later: still plagued by controversy.
Postgrad Med J 2000; 76:80-84 the Fellowship of Postgraduate Medicine, 2000.
11-Ferry BL, Misbah SA, Stephens P, Sherrell Z, Lythgoe H, Bateman E, Banner C, Jones J, Groome N. and Chapel HM.
Development of an anti-Salmonella typhi Vi ELISA: assessment of immunocompetence in healthy donors.
Clin Exp. Immunol. 136(2): 297-303, 2004.
12-Herath HM.
Early diagnosis of typhoid fever by the detection of salivary IgA.
J Clin Pathol. 56(9): 694-698, 2003.
13-Nandakumar KS, Palanivel V. and Muthukkaruppan V.
Diagnosis of typhoid fever: detection of Salmonella typhi porins-specific antibodies by inhibition ELISA.
Clin Exp Immunol. 94(2): 317-321. 1993.
14-Sippel J, Bukhtiari N, Awan MB, Krieg R, Duncan JF, Karamat KA, Malik IA, Igbal LM. and Legters L.
Indirect immunoglobulin G (IgG) and IgM enzyme-linked immunosorbent assays (ELISAs) and IgM capture ELISA for detection of antibodies
to lipopolysaccharide in adult typhoid fever patients in Pakistan.
J Clin Microbiol. 27(6): 1298-1302, 1989.
15-Nardiello S, Pizzella T, Russo M. and Galanti B.
Serodiagnosis of typhoid fever by enzyme-linked immunosorbent assay determination of anti-Salmonella typhi lipopolysaccharide
antibodies.
J Clin Microbiol. 20(4): 718-721, 1984.
16-Barrett TJ, Blake PA, Brown SL, Hoffman K, Llort JM. and Feeley JC.
Enzyme-linked immunosorbent assay for detection of human antibodies to Salmonella typhi Vi antigen.
J Clin Microbiol. 17(4): 625-627, 1983.
17-Beasley WJ, Joseph SW. and Weiss E.
Improved serodiagnosis of Salmonella enteric fevers by an enzyme-linked immunosorbent assay.
J Clin Microbiol. 13(1): 106-114, 1981.
18-Henrik Chart, Thomas Cheasty, Elizabeth de Pinna, Lisa Siorvanes, John Wain, Didar Alam, Qamarauddin Nizami, Zulfiqar Bhutta and E.
John Threlfall.
Serodiagnosis of Salmonella enterica serovar Typhi and S. enterica serovars Paratyphi A, B and C human infections
Journal of Medical Microbiology. 56, 1161-1166, 2007.
19-Nowsheen Hamid and S. K. Jain.
Characterization of an outer membrane protein of Salmonella that confers protection against typhoid.
Clin. Vaccine Immunol. 15(9): 1461-1471, 2008.
20-Losonsky GA, Ferreccio C, Kotloff KL, Kaintuck S, Robbins JB. and Levine MM.
Development and evaluation of an enzyme-linked immunosorbent assay for serum Vi antibodies for detection of chronic Salmonella
typhi carriers.
J Clin Microbiol. 25(12): 2266-2269, 1987.
21-Taylor DN, Harris JR, Barrett TJ, Hargrett NT, Prentzel I, Valdivieso C, Palomino C, Levine MM. and Blake PA.
Detection of urinary Vi antigen as a diagnostic test for typhoid fever.
J Clin Microbiol. 18(4): 872-876, 1983.
|
| |
| المجلد 6 ,
العدد 6
, شعبان 1433 - تموز (يوليو) 2012 |
|
|
|
