| المجلد 8 ,
العددان 1 و 2
, رمضان 1437 - تموز (يوليو) 2016 |
| |
| From Therapeutic Drug Monitoring to Drug Management: IMPDH Activity as Novel Surrogate Marker for Dose Adjustment of Mycophenolate in Immunosuppressive Therapy |
| من مناطرة الدواء العلاجية إلى التدبير الدوائي: فاعلية IMPDH كواصم جديد بديل لضبط جرعة الميكوفينولات في العلاج الكابت للمناعة |
Manfred Küpper1, 2 and Rana Hallak3
1) Arab International University, Faculty of Pharmacy, Ghabageb, Syria
2) Institute for Immunology and Genetics, Kaiserslautern, Germany
3) Syrian Private University, Faculty of Pharmacy, Damascus, Syria
|
| الملخص Abstract |
Background: Mycophenolic acid is a well established inhibitor of inosine-monophosphate-dehydrogenase (IMPDH) in immunosuppressive therapy. Adverse side effects require dose reduction in more than half of the patients, increasing the risk for acute rejection. Unlike for other immunosuppressants, measuring the trough level shows no relevant correlation to the risk of graft loss. Instead, we determined the residual IMPDH activity, aiming on suggesting trusted intervals for drug management in the context of dose-reduction.
Materials and Methods: IMPDH activity (pmol/s per pmol AMP) was measured in 276 stable patients after renal transplantation.
Results: A mean overall residual activity of 38.9 ± 45.9 pmol/s pmol AMP (median = 28 pmol/s pmol AMP) was derived from 2505 individual measurements; the 10th percentile equaled 5 pmol/s pmol AMP, the 90th percentile 78 pmol/s pmol AMP.
Conclusion: Measurement of IMPDH activity in renal transplantation patients adds valuable information on the degree of immunosuppression.
|
| الخلفية: يعرف حمض الميكوفينول Mycophenolic acid كمثبط لخميرة الاينوزين مونوفسفات ديهيدروجيناز (IMPDH) في المعالجات الكابتة للمناعة. ونظراً لما يملكه هذا المركب من تأثيرات جانبية ضارة، فإن اللجوء إلى تخفيض الجرعة يصبح قضية لامفر منها بالنسبة لأكثر من نصف المرضى، الأمر الذي يزيد بدوره من احتمال الرفض الحاد.هذا ولم يبد قياس التراكيز القاعدية الدنيا trough level أية علاقة تتناسب مع رفض الطعم graft loss. تم في هذه الدراسة تحديد فاعلية الاينوزين مونوفسفات ديهيدروجيناز المتبقية IMPDH activityresidual، بما يساعد على وضع فترات تعاقب موثوقة للتدبير والمراقبة الدوائية فيما يخص خفض الجرعة.
المرضى: قمنا بقياس فعالية IMPDH مقدرة بـ pmol/s لكل pmol AMP لدى 276 مريضاً عقب غرس الكلية.
النتائج: بلغ المتوسط الكلي للفعالية المتبقية والمشتقة من 2505 قياس فردي ماقيمته38.9 ± 45.9 pmol/s pmol AMP مع median= 28 pmol/s pmol AMP. هذا وقد بلغت شريحة الـ 10 المئوية ما قيمته 5pmol/s pmol AMP، مقابل 78pmol/s pmol AMP لشريحة الـ 90 المئوية 90th percentile.
الاستنتاج: يضفي قياس فعالية IMPDH لدى مرضى الكلية معطيات قيمة حول درجة الكبت المناعي.
|
| Introduction |
Mycophenolic-acid (MPA) is a selective, non-competitive inhibitor of Inosine-Monophosphate-Dehydrogenase (IMPDH) leading to the inhibition of the de-novo synthesis of guanosine-nucleotides. In human lymphocytes inhibition of IMPDH results in altered cellular proliferation with arrest in the S-phase of the cell cycle. Due to the absence of a salvage pathway, proliferating activated T-cells are severely affected by the inhibitory effects of MPA (1; 2; 3).
Since its introduction in immunosuppressive therapy more than ten years ago, Mycophenolate-Mofetil (MMF) is an established part of immunosuppressive therapy after renal transplantation. Still in the first publication of the landmark Tricontinental trial because of possibly dose-related side effects of the drug (CMV-infection, gastrointestinal disturbances, and increased cancer risk) the need for individualization depending on clinical course or other factors was mentioned (4).
Side effects of MMF are causing dose reductions in approximately 60% of the patients leading to a cumulative and increasing risk for acute rejection (5). In addition, gastrointestinal (GIT) side effects affect medical adherence of the patients with consecutive risk for graft failure (6). In addition, dose reductions of MMF are related to increased costs, mainly due to frequent hospitalization of the patients (7).
USRDS data of 3589 patients with MMF prescription and GIT complaints revealed that dosage reduction or discontinuation of mycophenolatemofetil in the first 6 months after diagnosis of GI complications was associated with significantly increased risk of graft failure and increased healthcare costs in adult renal transplant recipients (8). Another report from USRDS data of 3675 patients with gastrointestinal complications under MMF and subsequent dose reduction also disclosed an increased risk for graft loss after dose reduction or discontinuation of MMF (9).
Apart from the risk of graft rejection subsequent to dose reduction in response to side-effects, the dose has to be adjusted during the course of therapy since the risk of graft loss declines with time, while toxic effects of the immunosuppressive drugs increase (10).
The usefulness of pharmacokinetic measurements of MMF was shown in early studies stating that the Area-under the curve (AUC) of MMF is predictive of the likelihood of allograft rejection after renal transplantation in patients receiving mycophenolatemofetil(11).
MPA trough levels show relevant inter- and intraindividual variability especially in patients with elevated serum creatinine and proteinuria (12; 13; 14). Clinically important, low trough levels are associated with an increased frequency of rejection (15), whereas elevated MPA trough levels are related to an increased risk for infections (16). Nevertheless, relevant correlations between MPA trough levels and MPA-AUC values could not be detected. In part this is owed to the enterohepatic recirculation 6-12 hours after administration, resulting in increased mycophenolate levels (17).
Furthermore, concomitant immunosuppressive therapy has major influence on MPA pharmacokinetics. Administration of mycophenolic acid concomitantly with Cyclosporine (CsA) or Tacrolimus significantly reduced rejection at trough levels >1.3 ng/ml (18). Since CsA has an inhibitory effect on the reabsorption of mycophenolate metabolites in the intestine, patients on CsA therapy exhibit lower MPA trough levels (19; 20). Consequently, MPA trough levels increased after discontinuation of CsA resulting in almost a doubling of MPA trough concentrations (21). Rath and Küpper give a concise overview of the influence of concomitant immunosuppressive therapy on MPA trough levels (22).
Therefore the usefulness of measuring trough levels in routine care of renal transplant recipients is doubted (23; 24; 25). To facilitate therapeutic drug monitoring, different limited sampling strategies for adult and pediatric patients after renal transplantation were established (17;26-32). Yet, determination of the AUC is neither feasible in routine care since it requires at least three samples within two hours of administration. (31; 32; 33; 34; 35; 36; 37)
The need for dose adjustment and the described insufficiency of mycophenolate pharmacokinetics make a surrogate marker based on one point t0 measurement, with the same predictive value as the AUC, highly desirable. This report describes a novel pharmacodynamic approach which assesses the level of immunosuppression as a function of the residual activity of the IMPDH, the target enzyme of mycophenolate. It could already be demonstrated that patients with low IMPDH activity before renal transplantation experienced more MMF dose reductions within the first 3 years. In addition, patients with high IMPDH activity and MMF dose reduction showed the highest rejection rate (33).
The method described here has been applied on a total of 276 patients in the maintenance phase after renal transplantation. The data obtained from this large cohort are ranked into percentiles with the ultimate goal to suggest trusted intervals of IMDPH residual activity as a tool to assist decision-making about possible dose reduction.
|
| Materials and Methods |
IMPDH Activity Assay
The samples are processed and IMPDH activity is assessed as described elsewhere (34).
Patients
IMPDH activity was consecutively measured in a total of 276 maintenance renal transplant patients acquired from several centers as part of the routine follow-up.
|
| Results |
During a period of approx. 3 years measurements from 2432 individual trough levels and 73 kinetics were obtained from 276 maintenance renal transplant patients, making a total of 2651 consecutive measurements. IMPDH residual activity shows considerable interindividual variability, going together with data from literature (33; 35), with a mean residual activity of 38.9 ± 45.9 pmol/s pmol AMP (median = 28 pmol/s pmol AMP).
The individual trough level measurements plus the t0 values of the kinetics, making a total of 2505 values, were utilized to rank the residual activities into percentiles and to derive preliminary cut-off values for evaluating the level of immunosuppression in the context of dose-adjustment (
).
|
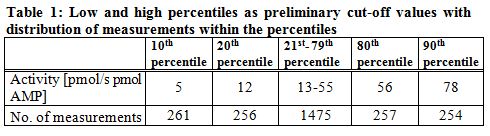 |
| The residual IMPDH activity of 10% of measurements was below 6 pmol/s pmol AMP (10th percentile = 5 pmol/s pmol AMP), the 20th percentile was calculated as 12 pmol/s pmol AMP. Twenty percent of activity values were equal to or above 56 pmol/s pmol AMP (80th percentile), and ten percent of assessed activities ranged above 77 pmol/s pmol AMP (90th percentile 78 pmol/s pmol AMP). |
| Discussion |
The motivation behind this work was the ultimate goal to establish trusted intervals for the residual IMPDH activity which, together with other parameters, would assist any decision concerning dose reduction or dose escalation. Any activity above such interval would indicate insufficient immunosuppression, requiring rather dose escalation, while residual activity below the trusted interval would plead for dose reduction, for possible adverse side effects like GIT, risk of infection or toxicity. For patients with activities within the trusted interval, dose reduction should be possible if indicated, without establishing an increased risk of graft loss.
Generally, it should be fair to assume that any residual activity below the tenth percentile indicates very strong immunosuppression, allowing for immediate dose reduction. In contrast, values above the 90th, possibly already above the 80th percentile should not allow for any dose reduction, or rather demand dose escalation in patients on lose-dose schemes. Especially long term patients, who received dose reduction according to the standard protocol could benefit from this.
Based on our data (n= 2505 measurements), the tenth percentile is defined as 5 pmol XMP/s pmol AMP, while the 80th percentile is marked by 56 pmol XMP/s pmol AMP, and the 90th percentile by 78 pmol XMP/s pmol AMP. If these values are regarded as preliminary cut-offs, they can give valuable information to the physician in the context of therapeutic drug management. Still, more correct limits of a trusted interval needed to be defined in course of a prospective, multi-centric study.
|
| Summary |
Therapeutic drug monitoring based on trough levels isn’t feasible for mycophenolic acid, since it’s not informative neither with respect to the risk of graft loss nor to the risk of adverse side effects. The total drug exposure (AUC) can be deduced from three successive measurements, a procedure which is precise enough, yet not acceptable for clinical routine.
The described method of directly measuring the residual activity of the target enzyme IMPDH reflects the immediate effect of the drug, and hence the level of immunosuppression. These values shall allow judging, whether the suppression of enzymatic activity is insufficient or too stringent, putting the patient at risk of graft loss, or adverse side effects, respectively, or whether it is within a ‘safe corridor’. The data presented in this work are preliminary and intended as a guideline; further work, in the ideal case within a prospective, multicentric clinical study, is needed in order to define generally accepted cut-off values.
|
| Acknowledgement |
|
This work has been carried out as part of a non-intervened multi-centric study initiated by Novartis Pharma GmbH, Nürnberg, Germany, and directed by the group of Prof. Dr. K. Budde, Dept. of Nephrology, Charitée, Berlin, Germany. All samples have been processed at the Institute for Immunology and Genetics, Kaiserslautern, Germany. The vast majority of samples were provided by Dr. Thomas Rath, MD, Assistant Medical Director at the Dept. of Nephrology and Transplantation Medicine, Medical Clinics III, Westpfalzklinikum Kaiserslautern, Germany, whom we’d like to thank for his collaboration and support. Further samples were acquired from the hospital BarmherzigeBrüder, Trier, Germany, the University Hospital Mainz, Germany, as well as from several private clinics.
|
| References |
1. Allison, AC und Eugui, EM. Purine metabolism and immunosuppressive effects of mycophenolate mofetil (MMF).
Ckin Transplant. Feb 1996, Bd. 10, 1 Pt2, S. 77-84.
2. Cohn, RG, et al.
Mycophenolic acid increases apoptosis, lysosomes and lipid droplets in human lymphoid and monocytic cell lines.
Transplantation. Aug 1999, Bd. 68, 3, S. 411-8.
3. Dayton, JS, et al.
Effects of human T lymphocyte activation on inosine monophosphate dehydrogenase expression.
J Immunol. Feb 1994, Bd. 152, 3, S. 984-91.
4. A blinded, randomized clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation. The Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group.
Transplantation. Apr 1996, Bd. 61, 7, S. 1029-37.
5. Knoll, G. A; et al.
Mycophenolate mofetil dose reduction and the risk of acute rejection after renal transplantation.
J Am Soc Nephrol. 2003, Bd. 14, 9, S. 2381-6.
6. Takemoto, S. K; et al.
A retrospective analysis of immunosuppression compliance, dose reduction and discontinuation in kidney transplant recipients.
Am J Transplant. 2007, Bd. 7, 12, S. 2704-11.
7. Tierce, J. C; et al. Impact of mycophenolate mofetil (MMF)-related gastrointestinal complications and MMF dose alterations on transplant outcomes and healthcare costs in renal transplant recipients.
Clin Transplant. 2005, Bd. 19, 6, S. 779-84.
8. Machnicki, G;et al.
Economic impact and long-term graft outcomes of mycophenolate mofetil dosage modifications following gastrointestinal complications in renal transplant recipients.
Pharmacoeconomics. 2008, Bd. 26, 11, S. 951-67.
9. Bunnapradist, S;et al.
Mycophenolate mofetil dose reductions and discontinuations after gastrointestinal complications are associated with renal transplant graft failure. Transplantation. 2006, Bd. 82, 1, S. 102-7.
10. Budde, K. und Glander, P.
Pharmacokinetic principles of immunosuppressive drugs. Ann Transplant. 2008, Bd. 13, 3, S. 5-10.
11. Hale, MD, et al. The pharmacokinetic-pharmacodynamic relationship for mycophenolate mofetil in renal transplantation.
Clin Pharmacol Ther. Dec 1998, Bd. 64, 6, S. 672-83.
12. Merkel, U;et al.
Trough levels of mycophenolic acid and its glucuronidated metabolite in renal transplant recipients.
Int J Clin Pharmacol Ther. 2005, Bd. 43, 8, S. 379-88.
13. Fernández, A; et al.
Mycophenolate mofetil levels in stable kidney transplant recipients.
Transplant Proc. 2007, Bd. 39, 7, S. 2182-4.
14. Fernández, A;et al.
Variability of mycophenolate mofetil trough levels in stable kidney transplant patients.
Transplant Proc. 2007, Bd. 39, 7, S. 2185-6.
15. Oellerich, M;et al.
Pharmacokinetic and metabolic investigations of mycophenolic acid in pediatric patients after renal transplantation: implications for therapeutic drug monitoring. German Study Group on Mycophenolate Mofetil Therapy in Pediatric Renal Transplant Recipients.
Ther Drug Monit. 2000, Bd. 22, 1, S. 20-6.
16. Smak Gregoor P. J;et al.
Unusual presentation of herpes virus infections in renal transplant recipients exposed to high mycophenolic acid plasma concentrations.
Transpl Infect Dis. 2003, Bd. 5, 2, S. 79-83.
17. Johnson, A. G;et al.
The kinetics of mycophenolic acid and its glucuronide metabolite in adult kidney transplant recipients.
Clin Pharmacol Ther. 1999, Bd. 66, 5, S. 492-500.
18. Gaston, R. S;et al.
Fixed or controlled-dose mycophenolate mofetil with standard or reduced dose calcineurin inhibitors: The Opticept Trial.
American Journal of Transplantation. 2009, Bd. 9, S. 1607-19.
19. Kuypers, D, et al.
Consensus report on therapeutic drug monitoring of mycophenolic acid in solid organ transplantation.
Clinical Journal of the American Society of Nephrology. 2010, Bd. 5, S. 341-58.
20. Smak Gregoor, P. J;et al.
Mycophenolic acid plasma concentrations in kidney allograft recipients with or without cyclosporin: a cross-sectional study.
Nephrol Dial Transplant. 1999, Bd. 14, 3, S. 706-8.
21. Gregoor, P. J;et al.
Effect of cyclosporine on mycophenolic acid trough levels in kidney transplant recipients.
Transplantation. 1999, Bd. 68, 10, S. 1603-6.
22. Rath, T. und Küpper, M.
Pharmacokinetics and Pharmacodynamics of Mycophenolate in Patients After Renal Transplantation.
Renal Transplantation - Updates and Advances. 1st edt. s.l. : Intech Open Access Publisher, 2011, 11, S. 163-78.
23. Mardigyan, V;et al.
Best single time points to predict the area-under-the-curve in long-term heart transplant patients taking mycophenolate mofetil in combination with cyclosporine or tacrolimus.
J Heart Lung Transplant. 2005, Bd. 24, 10, S. 1614-8.
24. Jirasiritham, S;et al.
The pharmacokinetics of mycophenolate mofetil in Thai kidney transplant recipients.
Transplant Proc. 2004, Bd. 36, 7, S. 2076-8.
25. Pape, L; Ehrich, J. H. und Offner, G.
Long-term follow-up of pediatric transplant recipients: mycophenolic acid trough levels are not a good indicator for long-term graft function.
Clin Transplant. 2004, Bd. 18, 5, S. 576-9.
26. Willis, C;et al.
Evaluation of limited sampling strategies for estimation of 12-hour mycophenolic acid area under the plasma concentration-time curve in adult renal transplant patients.
Ther Drug Monit. 2000, Bd. 22, 5, S. 549-54.
27. El Haggan, W;et al.
Pharmacokinetics of mycophenolic acid in kidney transplant patients receiving sirolimus versus cyclosporine.
Transplant Proc. 2005, Bd. 37, 2, S. 864-6.
28. Miura, M;et al.
Limited sampling strategy for simultaneous estimation of the area under the concentration-time curve of tacrolimus and mycophenolic acid in adult renal transplant recipients.
Ther Drug Monit. 2008, Bd. 30, 1, S. 52-9.
29. Mohammadpour, A. H;et al.
Estimation of abbreviated mycophenolic acid area under the concentration-time curve during early posttransplant period by limited sampling strategy.
Transplant Proc. 2008, Bd. 40, 10, S. 3668-72.
30. Filler, G.
Abbreviated mycophenolic acid AUC from C0, C1, C2, and C4 is preferable in children after renal transplantation on mycophenolate mofetil and tacrolimus therapy.
Transpl Int. 2004, Bd. 17, 3, S. 120-5.
31. Schütz, E;et al.
Limited sampling strategy for the determination of mycophenolic acid area under the curve in pediatric kidney recipients. German Study Group on MMF Therapy in Pediatric Renal Transplant Recipients.
Transplant Proc. 1998, Bd. 30, 4, S. 1182-4.
32. Weber, L. T;et al.
Therapeutic drug monitoring of total and free mycophenolic acid (MPA) and limited sampling strategy for determination of MPA-AUC in paediatric renal transplant recipients. The German Study Group on Mycophenolate Mofetil (MMF) Therapy.
Nephrol Dial Transplant. 1999, Bd. 14, Suppl 4, S. 34-5.
33. Glander, P;et al.
Pre-transplant inosine mono-phosphate dehydrogenase activity is associated with clinical outcome after renal transplantation.
Am J Transplant. 2004, Bd. 4, 12, S. 2045-51.
34. Rath, T. und Küper, M.
Comparison of Inosine-Monophosphate-Dehydrogenase (IMPDH) Activity in Patients With Enteric-Coated Mycophenolate-Sodium (EC-MPS) or Microphenolate Mofetil (MMF) After Renal Transplantation.
Transplant Proc. 2009, Bd. 41, 6, S. 2524-8.
35. Budde, K; Braun, K. P. und Glander, P.
Pharmacodynamic monitoring of mycophenolate mofetil in stable renal allograft recipients.
Transplant Proc. 2002, Bd. 34, S. 1748.
36. Musuamba, FT, et al.
Limited sampling models and Bayesian estimation for mycophenolic acid under the curve prediction in stable renal transplant patients co-medicated with cyclosporin or sirolimus.
Clinical Pharmakokinetics. 2009, Bd. 48, 11, S. 745-58.
37. van Gelder, T, et al.
Renal transplant patients at high risk of acute rejection benefit from adequate exposure to mycophenolic acid.
Transplantation. 2010, Bd. 89, 5, S. 595-9.
38. Arns, W;et al.
Enteric-coated mycophenolate sodium delivers bioequivalent MPA exposure compared with mycophenolate mofetil.
Clin Transplant. 2005, Bd. 19, 2, S. 199-206.
39. Budde, K;et al.
Pharmacokinetic and pharmacodynamic comparison of enteric-coated mycophenolate sodium and mycophenolate mofetil in maintenance renal transplant patients.
Am J Transplant. 2007, Bd. 7, 4, S. 888-98.
40. Johnston, A; He, X. und Holt, D. W.
Bioequivalence of enteric-coated mycophenolate sodium and mycophenolate mofetil: a meta-analysis of three studies in stable renal transplant recipients.
Transplantation. 2006, Bd. 82, 11, S. 1413-8.
41. Glander, P;et al.
Non-radioactive determination of inosine 5'-monophosphate dehydrogenase (IMPDH) in peripheral mononuclear cells.
Clin Biochem. 2001, Bd. 34, 7, S. 543-9.
42. Zucker, K;et al.
Unexpected augmentation of mycophenolic acid pharmacokinetics in renal transplant patients receiving tacrolimus and mycophenolate mofetil in combination therapy, and analogous in vitro findings.
Transpl Immunol. 1997, Bd. 5, 3, S. 225-32.
43. van Hest, R. M;et al.
Explaining variability in mycophenolic acid exposure to optimize mycophenolate mofetil dosing: a population pharmacokinetic meta-analysis of mycophenolic acid in renal transplant recipients.
J Am Soc Nephrol. 2006, Bd. 17, 3, S. 871-80.
44. Cattaneo, D;et al.
Pharmacokinetics of mycophenolate sodium and comparison with the mofetil formulation in stable kidney transplant recipients.
Clin J Am Soc Nephrol. 2007, Bd. 2, 6, S. 1147-55.
45. Kamar N;et al.
Questionnaire-based evaluation of gastrointestinal disorders in de novo renal-transplant patients receiving either mycophenolate mofetil or enteric-coated mycophenolate sodium.
Nephrol Dial Transplant. 2005, Bd. 20, 10, S. 2231-6.
46. Budde, K;et al.
Enteric-coated mycophenolate sodium: safe conversion from mycophenolate mofetil in maintenance renal transplant recipients.
Transplant Proc. 2004, Bd. 36, 2 Suppl, S. 524S-7S.
47. Sánchez-Fructuoso A;et al.
Use of mycophenolate sodium in
stable renal transplant recipients in Spain: preliminary results of the MIDATA study.
Transplant Proc. 2009, Bd. 41, 6, S. 2309-12.
48. Chan, L;et al.
Patient-reported gastrointestinal symptom burden and health-related quality of life following conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium.
Transplantation. 2006, Bd. 81, 9, S. 1290-7.
49. Robaeys, G;et al.
Successful conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium (myfortic) in liver transplant patients with gastrointestinal side effects.
Transplant Proc. 2009, Bd. 41, 2, S. 610-3.
50. van Gelder, T;et al.
A randomized double-blind, multicenter plasma concentration controlled study of the safety and efficacy of oral mycophenolate mofetil for the prevention of acute rejection after kidney transplantation.
Transplantation. 1999, Bd. 68, 2, S. 261-6.
51. Le Meur, Y;et al.
Individualized mycophenolate mofetil dosing based on drug exposure significantly improves patient outcomes after renal transplantation.
Am J Transplant. 2007, Bd. 7, 11, S. 2496-503.
|
| |
| |
| المجلد 8 ,
العددان 1 و 2
, رمضان 1437 - تموز (يوليو) 2016 |
|
|
|
